Keywords
chagas disease,Copalic acid,Leishmaniasis,Leishmanicidal activity,Structural Modification,Trypanocidal activity...How to Cite
(1)
Silva Parra, A. N.; Ferreira Soares Rocha, A. C.; da Silva Pavan, J. C.; da Silva Machado, A.; Miranda Rodrigues, V. J.; Albino dos Santos, D.; Magalhães Caldas, L. G.; de Albuquerque, S.; Gomes Heleno, V. C. Antiparasitary Potential of Natural and Semi-Synthetic Labdane Diterpenes. Orbital: Electron. J. Chem. 2025, 17, 315-321.




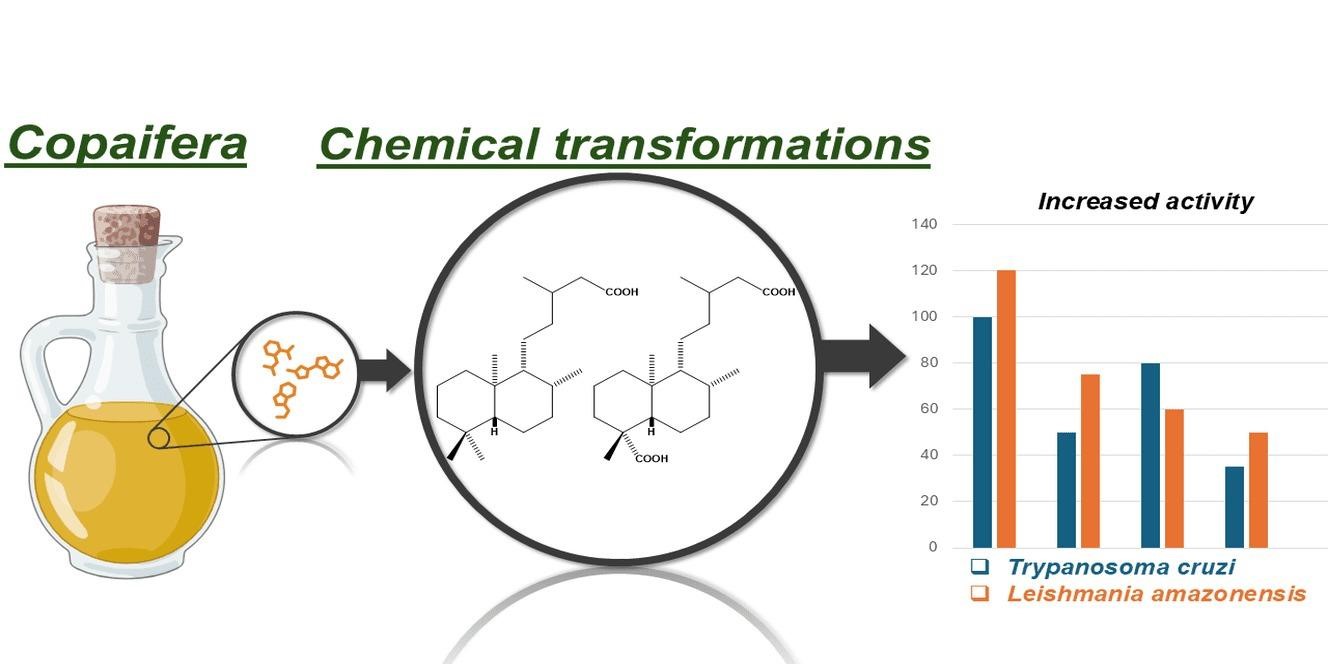
This paper describes the obtention of fourteen derivatives from four natural labdane diterpenes isolated from Copaifera oleoresin, named ent-copalic acid (1), ent-3b-acetoxy copalic acid (2), ent-3b-hydroxy copalic acid (3) and ent-agathic acid (4). All eighteen compounds, derivatives and precursors, were assayed against the promastigote form of Leishmania amazonensis and trypomastigote forms of Trypanosoma cruzi, revealing two promising compounds with leishmanicidal activity (IC50 = 5.94 mM and 5.31 mM) and three promising compounds with trypanocidal activity, two of them (IC50 = 13.31 mM and IC50 = 15.05 mM) displaying similar activity as the reference drug (IC50 = 13.12 mM) and one of them being even more potent with an IC50 = 0.425mM.
Comments (0)