Keywords
β-tubulin.,Carbendazim,Fusarium graminearum,Molecular Dynamics,QSARHow to Cite
(1)
Gasques, L. de S.; Morais, P. A. B.; Gonçalves , A. da S.; de Paula, H. Computational Methods As Screening Toll for Triazole Natural Products Derivatives As Potential β-Tubulin Inhibitors for Fusarium Graminearum. Orbital: Electron. J. Chem. 2025, 17, 322-338.




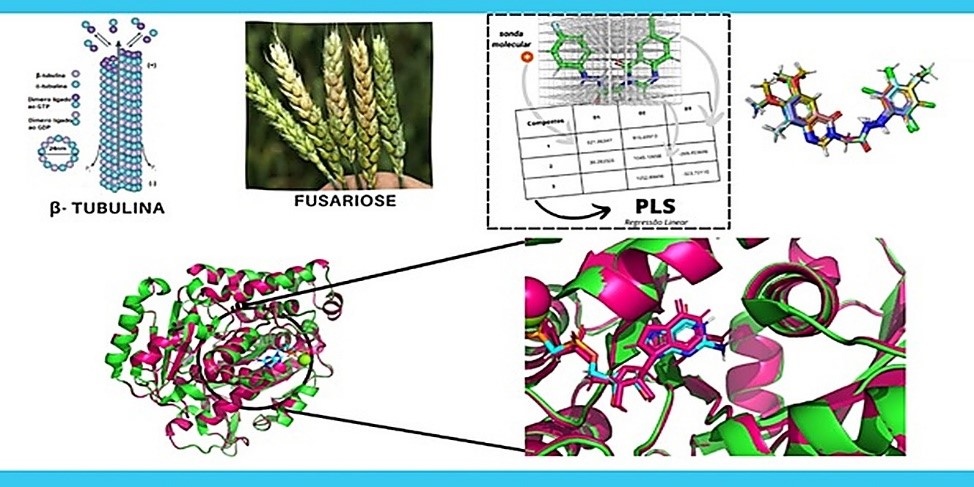
Phytopathogenic fungi like Fusarium graminearum are the cause of the disease gibberellosis, also known as Fusarium head blight (FHB). This disease shows significant losses in agriculture and effects on global economy. Nevertheless, this condition can be reduced with the use of commercial fungicides. Thus, this work describes computational studies performed on a group of natural products triazole derivatives using as starting point a small library of quinazolines synthesized by the literature. These quinazolines were applied to build QSAR models. Furthermore, using the Swiss Model server, a comparative modelling of the β-tubulin protein was realized to test the models and the compound 15, a triazole derivative of thymol successfully was further through both the QEPest and ProTox II toxicity tests. The behavior and stability between compound 15 and β-tubulin were then evaluated by 200 ns of molecular dynamics in comparison to carbendazim as standard inhibitor. Finally, the compound 15 revealed a stable complex with the protein throughout the simulation, exhibiting low fluctuations in the RMSF, indicating good interactions with the residues present. The investigation of the free energy of binding supports this, showing that whereas carbendazim had an MM/GBSA of -18 kcal/mol, thymol-derived 15 had an MM/GBSA of -44 kcal/mol.
Comments (0)