The emergence of antimicrobial-resistant bacteria has become a global health crisis, posing significant challenges to modern medicine 1. Among these pathogens, Staphylococcus aureus is the most prevalent human pathogen, causing a wide range of infections from mild skin and soft tissue infections to life-threatening conditions such as pneumonia, endocarditis, and device-associated infections 2. S. aureus is a commensal organism, colonizing the anterior nasal passages of 20% to 80% of the human population 3. However, its ability to rapidly develop resistance to a broad spectrum of antimicrobial compounds has made it a major concern in clinical settings 4. The U.S. Centre for Disease Control reports S. aureus as the second most prevalent pathogenic bacteria, highlighting its significance in public health.
Historically, S. aureus antimicrobial resistance is marked by the widespread use of methicillin and semi-synthetic anti-staphylococcal penicillin in the 1960s that led to the emergence of methicillin-resistant S. aureus (MRSA) 5. Since then, MRSA has become a leading hospital-associated pathogen 6, 7, 8, 9. Vancomycin, long considered the drug of last resort for severe MRSA infections, has shown decreased efficacy with the emergence of vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) in some regions 10. S. aureus has developed resistance to multiple antibiotic classes, including aminoglycosides, penicillin, macrolides, and tetracycline 11, leading to frequent outbreaks and treatment challenges 12.
Among the various known antibiotic resistance mechanisms, efflux pumps play a crucial role in S. aureus. These membrane-bound transporters act as critical defence mechanisms by expelling antibiotics and other toxic compounds from the bacterial cell, thereby reducing their intracellular concentration and efficacy 13. Recent studies have also suggested a link between efflux pumps, virulence and biofilm formation, highlighting their multifaceted role in S. aureus pathogenesis 14.
Biofilm formation represents a sophisticated survival strategy employed by S. aureus and other pathogens 15, 16, 17. In natural and clinical environments, S. aureus forms complex microbial communities encased in a protective extracellular matrix 18. This mode of growth significantly enhances S. aureus tolerance to various antibiotic classes, promotes persistence, and complicates treatment strategies. Studies have shown that efflux pumps contribute to biofilm formation by facilitating the export of quorum-sensing molecules necessary for biofilm development, enhancing tolerance to antimicrobial compounds within the biofilm structure and modulation of gene expression involved in extracellular matrix production 13, 19, 20.
This review presents an understanding of the role of efflux pumps in developing antibiotic resistance in S. aureus biofilm which will help in the development of effective therapeutic strategies against efflux pumps to overcome the increasing problem of biofilm associated infections.
ANTIBIOTIC RESISTANCE MECHANISMSAntibiotics are designed to eliminate microorganisms that are harmful to human health. However, the increased use of antibiotics has led to the emergence of resistant bacteria, suggesting that such organisms are present in the environment and may have evolved due to antibiotic exposure 13, 19, 20. S. aureus has developed resistance to various antibiotics. The mechanisms of antibiotic resistance in S. aureus are diverse and can be broadly categorized into intrinsic and acquired resistance. Acquired antibiotic resistance often results from plasmid-mediated resistance 21, mutations in chromosomal genes or by acquisition of external genetic elements of resistance 21, 22. MRSA exemplifies acquired resistance, primarily encoded by the mecA gene carried on the Staphylococcal Cassette Chromosome mec (SCCmec). This genetic element can be transferred between staphylococcal species, thus contributing to the spread of resistance 13.
S. aureus utilizes various mechanisms to develop intrinsic antimicrobial resistance, including limiting drug uptake, modifying drug targets, enzymatically inactivating drugs and actively effluxing the drugs 23, 24. Among these mechanisms, the efflux system is a major mode of intrinsic drug resistance in S. aureus and is described in detail in the following section.
KNOWN STRUCTURE AND FUNCTION OF EFFLUX PUMPS IN S. AUREUSEfflux pumps are ubiquitous membrane proteins involved in the export of the harmful substances from bacterial cells to the external environment 25. These proteins, either present on bacterial chromosomes or on plasmids 26 are employed by S. aureus, a key mechanism adopted to cope with the diverse range of antimicrobials used to treat infections 27.
Based on energy requirements and structure, efflux pumps in S. aureus are classified into five membrane protein families: Major Facilitator Superfamily (MFS), Small Multidrug Resistance (SMR), Multidrug and Toxin Extrusion (MATE) family, Resistance Nodulation Cell Division (RND) superfamily, and ATP-binding Cassette (ABC) superfamily (Figure 1) 28. MFS and SMR transporters utilize proton motive force to drive substrate extrusion via an anti-port H+ drug mechanism. The MATE family can use the sodium membrane gradient, while the ABC superfamily uses ATP hydrolysis to drive substrate extrusion 29.
–
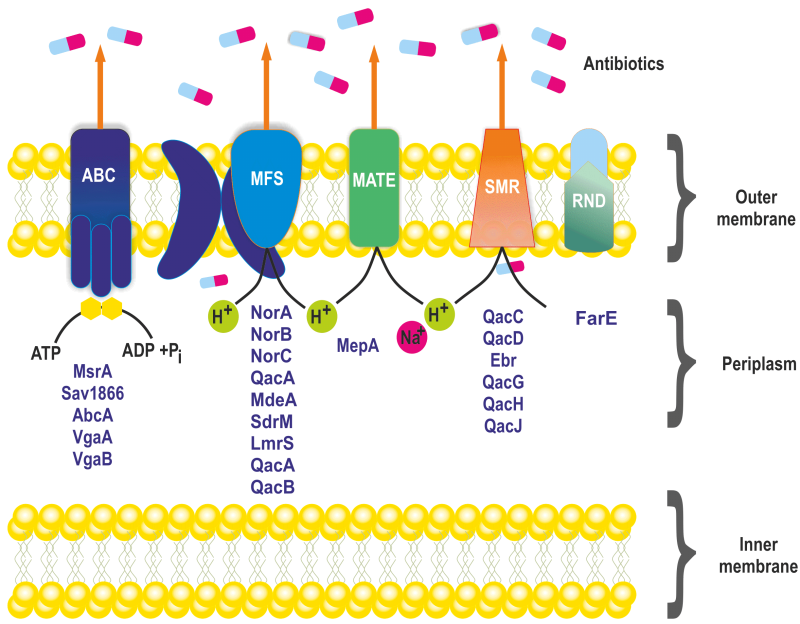
FIGURE 1: Known classes of multidrug efflux pumps in S. aureus. Multidrug efflux pumps identified in S. aureus are categorized into five families of membrane proteins: ATP binding cassette (ABC) superfamily, major facilitator superfamily (MFS), multidrug and toxin extrusion (MATE) family, small multidrug resistance (SMR) family and FarE, a resistance -nodulation division (RND) type efflux pumps.
Among the known efflux transporters in S. aureus, the MFS is the primary class, encoded by NorA, NorB, NorC, MdeA, SdrM, LmrS, QacA, and QacB efflux proteins (Table 1) 30. NorA is the most studied and predominant efflux pump associated with the first line defence against antimicrobials in S. aureus. It is often overexpressed in MRSA strains 31, 32. The NorA protein consists of twelve transmembrane segments with 388 amino acids, sharing 44% identity to Escherichia coli’s tetracycline efflux pump TetA and is 24% identical with Bacillus subtilis Bmr 33. NorA pumps expel a variety of compounds, including hydrophilic fluoroquinolones such as norfloxacin and ciprofloxacin, dyes like ethidium bromide, and biocides like quaternary ammonium compounds 34.
Table 1. Multidrug resistantefflux pumps reported in S. aureus.
Efflux pump family
Gene
Gene location
Regulator
Substrate
Reference
SMR
Smr (QacC, QacD, Ebr)
QacG, QacH
QacJ
Plasmid
Not known
Benzalkonium Chloride, Cetrimide, Chlorhexidine Diacetate, Ethidium Bromide, Proflavine, Cetyltrymethylammonium
MATE
MepA
Chromosome
MepR
Ciprofloxacin, Norfloxacin, Moxifloxacin Sparfloxacin Tigecycline, Pentamidine, Cetrimide, Benzalkonium Chloride, Dequalinium Tetraphenylphosphonium, Chlorhexidine, Ethidium Bromide, Acriflavine, Crystal Violet, Hoechst 33342, 4-6-Diamidino-2-Phenylindole
MFS
NorA, NorB,
NorC
Chromosome
MgrA,
NorG
Hydrophilic Fluoroquinolones (Ciprofloxacin, Norfloxacin, Dparofloxacin, Gemifloxacin, Premafloxacin), Qacs (Tetraphenyl Phosphonium, Benzalkonium Chloride), Cetrimide, Tetraphenyl Ammonium, Dyes (E.G. Ethidium Bromide, Rhodamine)
43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54
MdeA
Chromosome
Not known
Virginiamycin, Novobiocin, Mupirocin, Fusidic Acid, Doxorubicin, Daunorubicin, Benzalkonium Chloride, Tetraphenylphosphonium, Ethidium Bromide, Hoechst 33342
SdrM
Chromosome
Not known
Norfloxacin, Acriflavine, Ethidium Bromide
LmrS
Chromosome
Not known
Linezolid, Chloramphenicol, Florfenicol, Thrimethoprim Erythromycin, Kanamycin, Fusidic Acid, Lincomycin, Streptomycin Tetraphenylphosphonium, Ethidum Bromide
QacA/B
Plasmid
QacR
Pentamidine, Benzalkonium Chloride, Cetrimide, Chlorhexidine, Ethidium Bromide (Over 30 Mono And Divalent Cations). Benzalkonium Chloride, Tetraphenylphosphonium, Ethidium Bromide, Acriflavine, Rhodamine
TetA (K)
Tet38
Plasmid
TetR
MgrA
Tetracycline, Omadacycline, Amino Methylcycline, Tunicamycin, Fosfomycin, Fatty Acids
SepA
Chromosome
Not known
Benzalkonium Chloride, Chlorhexidine Gluconate, Dye Acriflavine
ABC
Sav1866
AbcA
Chromosome
Not known SarA
Doxorubicin, Vinblastine, Ethidium Bromide, Hoechst 33342, Oxacillin, Imipenem, Nafcillin, Penicillin G, Methicillin, Cefotaxime, Moenomycin Tetraphenylphosphonium, Rhodamine, Ethidium Bromide
44, 55, 56, 57, 58, 59, 60, 61
MsrA, VgaA
Vga(A) LC
VgaB
Plasmid
SarZ
MgrA
NorG
Not known
Erythromycin, Macrolides, Type B Streptogramins; Type A Streptogramin. Lincomycin, Clindamycin; Type A Streptogramin
NorB efflux pumps have structural similarities with Blt (41%), and Bmr (30%) of B. subtilis and NorA (30%), QacA (39%) of S. aureus. NorB confers resistance to a diverse range of antimicrobial compounds, including biocides like cetrimide, tetraphenylphosphonium, and dyes such as ethidium bromide, and hydrophobic and hydrophilic fluoroquinolones like norfloxacin and ciprofloxacin 43. The NorC efflux pump is comprised of 462 amino acids having twelve transmembrane domains and shares 61% similarity with the norB efflux gene of S. aureus 43. NorC is associated with low-level resistance to ciprofloxacin, moxifloxacin, garenoxacin, and the dye rhodamine. The Nor efflux pumps (NorA, NorB, NorC) are regulated by the global regulator MgrA. MgrA acts as a positive regulator for norA gene expression but a negative regulator of norB and norC gene expression (Table 1) 62, 63. This differential regulation of Nor efflux pumps by MgrA enables the bacterium to modulate its efflux pump expression in response to diverse environmental stressors and antimicrobial agents, thereby optimizing its survival and resistance strategies.
The MdeA efflux pump is a 479 amino-acid protein with 14 transmembrane segments shared similarities with LmrB of B. subtilis (24%), EmrB of E. coli (24%), and QacA of S. aureus (23%) 46. It confers resistance to biocides like benzalkonium chloride, dequalinium, and tetraphenylphosphonium, and dyes like ethidium bromide, and antibiotics such as virginiamycin, novobiocin, mupirocin, and fusidic acid 46, 61.
SdrM is a 447-amino-acid protein with 14 transmembrane segments, shows 23% similarity with NorB and 21% similarity with the QacA protein. The SdrM transporter confers resistance to antimicrobials like norfloxacin and dyes such as acriflavine and ethidium bromide.
The lincomycin resistance protein LmrS from S. aureus is 480 amino acids long with 14 putative membrane-spanning domains. It shows similarity with ImrB from B. subtilis (39%), farB from Neisseria gonorrhoeae (25%), and emrS from E. coli (3%). Antibiotics such as linezolid, tetraphenylphosphonium chloride, sodium dodecyl sulphate, trimethoprim, and chloramphenicol are less likely to be removed by LmrS efflux pump (Table 1) 48.
QacA and QacB are 514-amino-acid proteins with 14 transmembrane segments 64. QacA mediates resistance to antimicrobials such as ethidium bromide and rhodamine, quaternary ammonium compounds like benzalkonium chloride, tetraphenylphosphonium, and dequalinium, diamidines such as pentamine and DAPI, biguanides like chlorhexidine, and guanyl hydrazones 49, 52. On the other hand, plasmid-encoded QacB pump provides protection against monovalent lipophilic cations 64.
S. aureus TetA(K) and Tet38 efflux pumps mediate high levels of tetracycline resistance 65. The TetA(K) efflux gene is plasmid-encoded, functioning as a Na+(K+)/H+ antiporter comprising 459 amino acids and 14 transmembrane regions 66. Antibiotics such as tetracycline, oxytetracycline, and chlortetracycline are rendered ineffective by TetK, but to a lesser extent to minocycline, doxycycline, and 6-methyl-6-deoxytetracycline. Bacteria equipped with the TetK efflux pump are also resistant to extrinsic stimuli such as sodium stress, alkali stress, and potassium deficiency stress 67. Like the NorB efflux pump, TetK contributes to S. aureus colonisation on mouse skin and survival during abscess development 68. Chromosomally encoded Tet38 with 450 amino acids and 14 transmembrane domains 68, 69 shares 46% similarity with S. aureus tetK and 45% similarity with B. subtilis tetA 70. Tet38 is negatively regulated by MgrA, which confers resistance to quinolones and tetracyclines 71. Studies have shown that Tet38 also plays a role in S. aureus internalization into host cells through interaction with the CD36 receptor 72, suggesting its potential role in biofilm formation and host-pathogen interactions.
Another MFS efflux pump, SepA, comprising 157 amino acids is also encoded by the S. aureus genome. It confers low-scale resistance to antiseptics such as benzalkonium chloride, chlorhexidine gluconate, and chromosomal-encoded dye acriflavine (Table 1) 54.
The SMR transporters are episome encoded 36 comprised of 110 amino acids and possess four transmembrane helices 73. The SMR transporters include QacC (QacD, Ebr, or Smr), QacG, QacH, and QacJ. Despite differences in amino acid sequences, Smr and QacG/H/J have similar substrate specificities 36. This efflux pump confers resistance to quaternary ammonium compounds, such as benzalkonium chloride, and monovalent cationic dyes, such as ethidium bromide 74.
The MATE family member MepA efflux pump consists of 452-amino-acid protein having twelve transmembrane regions and is located on chromosome 2. It shares 26% identity with MATE transporters belonging to other organisms: CdeA from Clostridium difficile and NorM from Vibrio parahaemolyticus 75.
MepA is associated with a multidrug-resistant phenotype in clinical S. aureus strains, providing minimal resistance against ethidium bromide, chlorhexidine, pentamidine, tetraphenylphosphonium, quaternary ammonium compounds, fluoroquinolones (ciprofloxacin, norfloxacin), benzalkonium chloride, cetrimide, dequalinium, tetraphenylphosphonium, chloroquine, and tigecycline 40, 76, 77, 78. The mepA gene is controlled by MepR, which belongs to the MarR family of transcriptional repressors 39, 79.
S. aureus possess two chromosomally encoded ABC transporter, Sav1866 and abc. These transporters are single polypeptides possessing transmembrane and nucleotide-binding domains that, upon dimerization, produce a functional transporter. The crystal structure of Sav1866 has been identified 55. Functional studies have shown that Sav1866 can transport diverse substrates such as ethidium bromide, Hoechst 33342, tetraphenylphosphonium, verapamil and vinblastine 56. The other plasmid encoded ABC transporter belonging to the MsrA efflux pumps and possessing a single-nucleotide-binding-domain may interact with other transmembrane proteins 59. In addition, the Vga proteins are also expressed by genes present on plasmids with a ATP-binding-domain (ABD) transporters (Table 1, Figure 2) 80, 81.
–
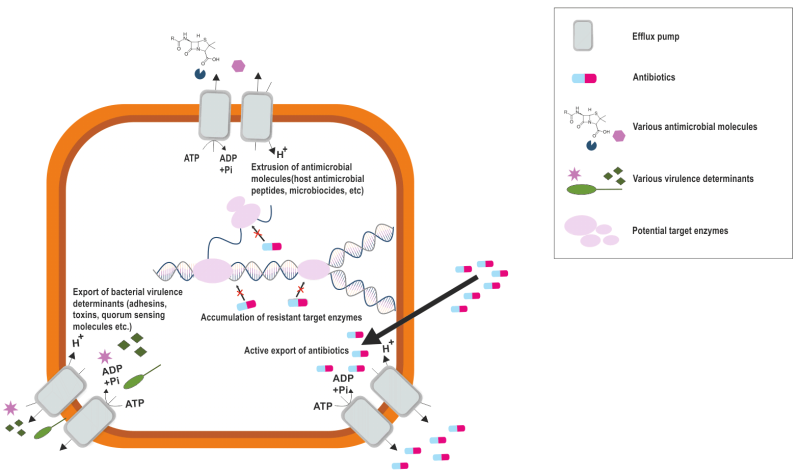
FIGURE 2: Intrinsic and antibiotic induced implications of bacterial efflux pumps. Bacterial efflux pumps function by (1) increasing bacterial pathogenicity by extruding antibacterial molecules produced from the host and secreting bacterial virulence factor, (2) reducing antibiotic efficacy by pumping them out of the bacteria, thus lowering intracellular concentration of antibiotics, which can increase development of further resistance.
The role of efflux pumps is widely known in antibiotic resistance, however, they regulate the internal environment by extruding toxic substances, biofilm formation molecules, quorum sensing molecules and virulence factors (Figure 2) 13. This multifaceted role of efflux pumps underscores their importance not only in antibiotic resistance but also in the overall pathogenicity and survival strategies of S. aureus.
BIOFILM FORMATION IN S. AUREUSBiofilms are complex, sessile microbial communities encased in a self-produced extracellular polymeric substance (EPS) that adheres to surfaces and forms aggregates 82. The EPS, comprised of polysaccharides, proteins and nucleic acids, constitutes up to 90% of the biofilm’s dry weight and provides the immediate environment for the microorganisms to form a biofilm 83, 84. The interaction between EPS and bacterial aggregates confers cohesion and viscoelasticity to the biofilm structure 85.
Like most of the bacterial species, S. aureus possesses similar stages of biofilm development, namely, attachment, accumulation, and detachment 86. During the attachment stage, S. aureus initiates biofilm formation through the adhesion of planktonic cells to natural or biomaterial surfaces. This event is mediated through the organization of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). The major components of MSCRAMM includes fibronectin-binding proteins (FnbA and FnbB) 87, clumping factors such as ClfA, ClfB 88, and serine-aspartate repeat family proteins such as SdrC, SdrD and SdrE 89.
Following initial attachment, bacterial cells proliferate and begin to produce EPS in response to environmental cues 90. During the aggregation stage, bacteria form biofilms by recognizing environmental signals that stimulate intracellular signal molecules and regulatory networks which leads to the proliferation and thickening of the biofilm 91. Thus, a compact three-dimensional mushroom like structure of the formed biofilm is encased in a extracellular matrix which provides resistance against human immune system and antibiotics 92.
As the biofilm matures, dispersal of the biofilm is triggered leading to the release of cells from the biofilm 93. This stage is important for colonization of new surfaces, dissemination of infection and continuation of the biofilm life cycle (Figure 3).
–
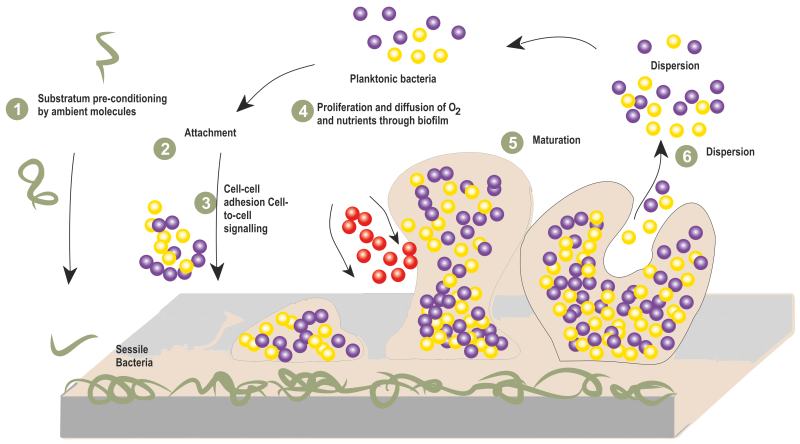
FIGURE 3: Steps involved in biofilm formation of S. aureus.
Despite being susceptible to antibiotics, bacteria have an inherent capacity to survive, which occurs by forming a sessile community called biofilms 94. The role of efflux pumps in biofilms is known to be functioning through excretion of extracellular matrix molecules and quorum sensing molecules that mediate biofilm formation, in addition to effluxing the harmful molecules and influencing the surface adhesion 13. The expression of efflux genes in S. aureus biofilms has been the determining factor for efflux pump mediated resistance. A study reported that the expression of several efflux and transporter genes was altered during biofilm growth compared to exponential and stationary phase cells 95. A comparative transcriptomic study on S. aureus cells under planktonic and biofilm conditions showed that the expression of transporter genes was higher in biofilms than in planktonic cells 96. Of the MFS transporters mdeA, norB, and norC, which are upregulated in S. aureus biofilms, norB and norC pumps extrude cetrimide, ethidium bromide, quinolones, and tetraphenylphosphonium and the mdeA efflux pump exports a range of quaternary ammonium compounds and antibiotics46, 97. These observations thus suggest that norB gene expression is upregulated in response to acid shock but reduced under other conditions, thus suggesting that it may be involved in maintaining low pH in the biofilm 75. Also, the norB gene functions to ensure that biofilm cells are protected from the toxic effects of organic acids produced during anaerobic respiration 98. Studies demonstrated that MgrA, a pleiotropic regulator of S. aureus, acts as a negative regulator for the norB and norC efflux gene, repressing the biofilm formation and thus establishing a link between efflux pump and biofilm formation in S. aureus 71. Moreover, a hypothetical gene showing characteristics of the MFS family was identified in an insertional mutant library in a high biofilm-forming clinical isolate of S. aureus, which, when disrupted, led to a defective biofilm 99. Although several efflux pumps have been described, most of the studies explain the role of MFS type efflux pumps in biofilm formation of S. aureus. However, the exact mechanism of efflux pumps in mediating antibiotic resistance in biofilms, the role of non-MFS efflux pump families in biofilm formation and maintenances and the potential of targeting efflux pumps as a strategy to combat biofilm-associated infections remains elusive in S. aureus.
EFFLUX PUMPS INHIBITORSThe emergence of antimicrobial resistance among clinical strains of S. aureus has necessitated the development of novel therapeutic strategies. Efflux pump inhibitors (EPIs) have emerged as a promising approach to combat antibiotic resistance. EPIs function by blocking the extrusion of antibiotics, thereby restoring antimicrobial susceptibility and enhancing the clinical efficacy of the existing antibiotics
Comments (0)