Tuberculosis (TB) is an infectious disease transmitted through aerosols and is caused by Mycobacterium tuberculosis (MTB). In 2022, approximately 10.6 million people fell ill with TB, and caused 1.3 million deaths around the world 1. Although both innate and adaptive immunity are activated in the immune responses related to TB, MTB employs several immune evasion strategies to resist host immunity and establish infection 2, 3, 4, 5.
Decreased T-cell function following MTB infection has been associated with impaired antigen presentation and reduced expression of MHC II and co-stimulatory molecules 6, 7. Immune checkpoint molecules such as Programmed Death 1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) are involved in regulating T cell function 8, 9. Increased expression of Programmed Death-Ligand 1 (PD-L1) has been observed in peripheral blood mononuclear cells (PBMCs), pleural effusion mononuclear cells (PEMC), and lung tissues of patients with active TB 10, 11. Activation of the PD-1/PD-L1 pathway has been associated with a higher bacterial burden in patients with TB, and PD-1/PD-L1 expression decreases following effective anti-TB treatment 12, 13. These findings suggest a potential role for the PD-1/PD-L1 pathway in the pathogenesis of MTB infection.
The promoter methylation of PD-L1 regulates its expression in patients with various types of malignancies and has been reported as an independent factor associated with treatment outcomes 14. DNA methyltransferases (DNMTs) and ten eleven translocation (TET) methylcytosine dioxygenases are the enzymes involved in DNA methylation. Increased expression of PD-1 and PD-L1 is a well-documented characteristic of immune responses related to MTB, and may contribute to T cell exhaustion. Our previous work well-demonstrated that the expression of PD-L1 is increased in CD14+ monocytes isolated from PBMC of patients with active TB 13. The expression of PD-L1 correlates with MTB bacterial burden and declines after initiation of anti-TB treatment. However, few studies have investigated the modulatory effects of promoter methylation on PD-L1 expression in patients with active TB. Therefore, we hypothesized that promoter hypomethylation enhances PD-L1 expression in patients with MTB infection and is associated with their clinical presentation and treatment outcomes. In this study, we prospectively enrolled patients with active TB and examined the association between methylation status and PD-L1 expression. Findings from patients with active TB were further validated through in vitro experiments.
RESULTSPatient characteristics
During the study period, 120 individuals were prospectively enrolled, including 80 patients with active TB and 40 non-TB subjects. The demographic characteristics of the enrolled patients are shown in Table 1. The mean age was 62.7 ± 17.6 years in patients with TB and 52.3 ± 17.6 years in the non-TB subjects (p = 0.003). The body mass index (BMI) was 21.4 ± 3.2 in patients with TB and 23.1 ± 3.0 in non-TB subjects (p = 0.007). Among patients with TB, 31.6% had comorbid diabetes, and 10.1% had inactive malignancies. Extrapulmonary TB was present in 14 (17.5%) patients with TB, and sputum smear positivity was observed in 21 (26.3%) of them.
Table 1. Demographic characteristics and diseaseseverities of active TB patients and non-TB individualsa.
Case number
80
40
Mean age (SD)
62.7 (17.6)
52.3 (17.2)
0.003
Male sex
52 (6%)
20 (50%)
0.114
BMI (SD)
21.4 (3.2)
23.1 (3.0)
0.007
Smoking history
36 (45.6%)
6 (15%)
0.001
Prior TB history
5 (6.3%)
0
0.040
Diabetes
25 (31.6%)
3 (7.5%)
0.003
Renal insufficiency
3 (3.8%)
0
0.114
COPD
5 (6.3%)
1 (5%)
0.672
Malignancy
8 (10.1%)
3 (7.5%)
0.635
Autoimmune disorders
6 (7.6%)
1 (2.5%)
0.264
TB site
–
Pulmonary TB
66 (82.5%)
–
Extrapulmonary TB
14 (17.5%)
–
Sputum smear status
–
Smear positive
21 (26.3%)
–
Smear negative
59 (73.8%)
–
Increased expression of PD-L1 and demethylation-related genes in TB patients
The expression levels of PD-L1, DNMT1, DNMT3A, DNMT3B, TET1, and TET2 in PBMCs obtained from patients with active and no TB are illustrated in Figure 1A. The results revealed that patients with active TB exhibited elevated expression of PD-L1, DNMT3B, TET1, and TET2, while showing decreased expression of DNMT1 compared to that in the non-TB subjects. We also investigated the expression of these genes in patients with active TB before and after the anti-TB treatment. As shown in Figure 1B, after initiating the anti-TB treatment, PD-L1 and TET1 expression was significantly decreased. We also observed a trend towards increased DNMT1 expression after the initiation of anti-TB treatment.
–
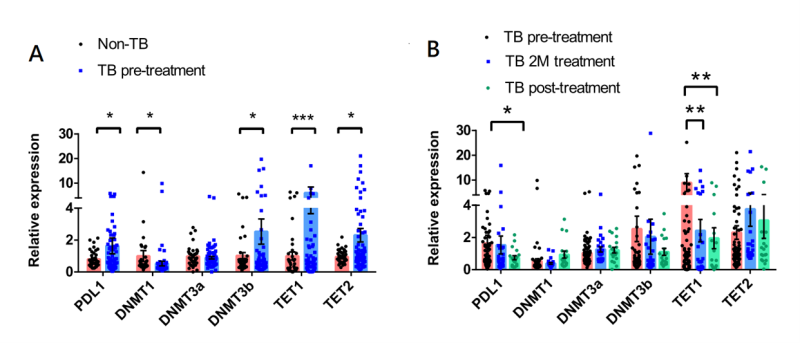
FIGURE 1: Comparisons of PD-L1, DNMTs, and TETs expression in peripheral blood mononuclear cells between (A) patients with active TB and non-TB subjects, and in (B) patients with active TB patients upon TB diagnosis and after anti-TB treatment. The data in bar charts are mean values of relative expression compared to GAPDH as reference. Differences between groups were compared using the Mann–Whitney U test. PD-L1 - programmed death-ligand 1; DNMTs - DNA methyltransferases; TETs - ten eleven translocation methylcytosine dioxygenases; 1M - 1-month after anti-TB treatment; 2M - 2-month after anti-TB treatment.
We conducted further investigations to explore the correlation between the expression levels of PD-L1, DNMT1, DNMT3A, DNMT3B, TET1, and TET2, and the clinical characteristics of patients with active TB. The results are shown in Figure 2. Patients with active TB and fever as well as positive sputum smears exhibited significantly higher expression of PD-L1 than in those without fever and with negative sputum smears. Patients with weight loss displayed lower DNMT1 expression. Moreover, patients with extrapulmonary involvement exhibited lower TET1 expression than in those with pulmonary TB. In terms of treatment outcomes, as illustrated in Figure 2, patients who did not achieve smear and culture conversion after one month of treatment showed significantly higher expression of PD-L1 and TET1 than in those who achieved conversion.
–
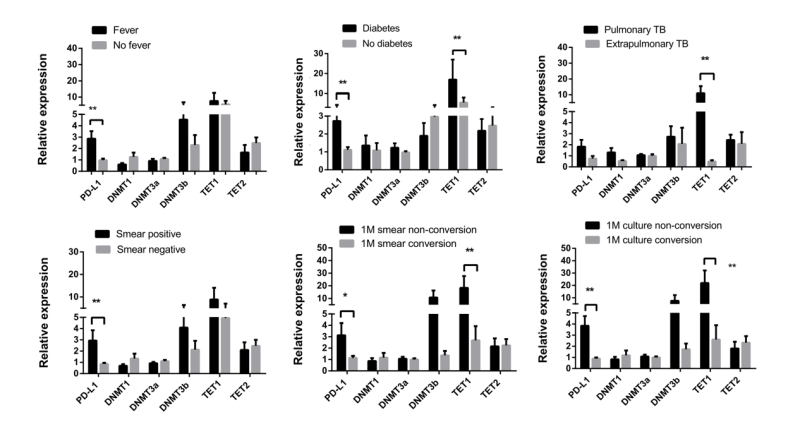
FIGURE 2: Comparisons of PD-L1, DNMTs, and TETs expression in PBMCs from patients with active TB with various clinical presentations and treatment outcomes. The data in bar charts are mean values. Error bars are standard errors. Differences between groups were compared using the Mann– Whitney U test. PD-L1 - programmed death-ligand 1; DNMTs - DNA methyltransferases; TETs - ten eleven translocation methylcytosine dioxygenases.
Increased expression of PD-L1 and TET1 in lung tissues and macrophages
Lung tissues were surgically resected from two patients with active pulmonary TB. Both lung tissues exhibited granulomatous inflammation and were tested positive for MTB in culture. Immunohistochemistry (IHC) staining revealed co-localization of TET1 and PD-L1 positive cells within inflammatory tissues (Figure 3). These cells were primarily positive for CD68, indicating that they were macrophages.
–
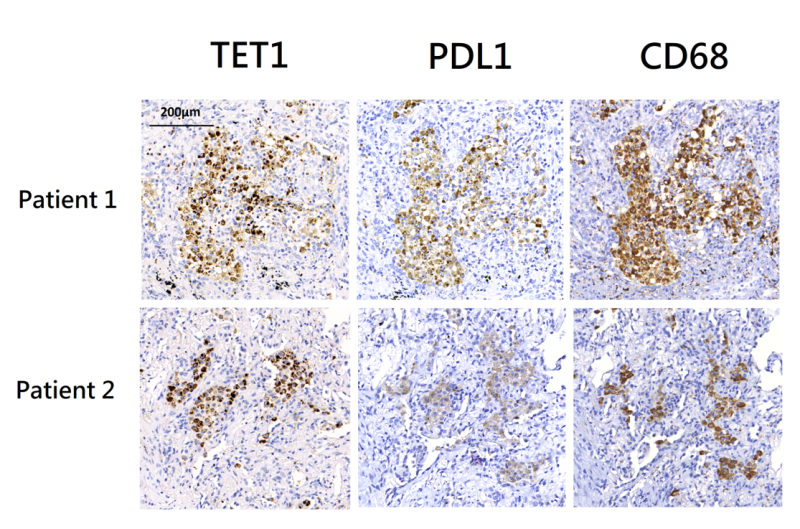
FIGURE 3: Immunohistochemistry (IHC) staining of lung tissues from two patients with pulmonary tuberculosis. IHC staining using anti-PD-L1 and anti-TET1 antibodies showed the presence of PD-L1 and TET1 positive cells. It also demonstrated the co-localization of PD-L1 and TET1. IHC staining using an anti-CD68 antibody suggested that these PD-L1/TET1 positive cells were primarily macrophages. PD-L1 - programmed death-ligand 1; TET1 - ten eleven translocation methylcytosine dioxygenases.
We conducted immunofluorescence (IF) analysis of THP-1 cells stimulated with MTB-related proteins. Upon treatment with MTB whole cell lysate (H37Rv, 10 μg/ml) and EsxA protein (1 μg/ml), TET1 and PD-L1 expression was increased in THP-1 cells for both stimulations (Figure 4). In the merged images, immunoreactivity towards the TET1 antibody overlapped with the PD-L1 staining. These findings suggest that human macrophages with high PD-L1 expression also exhibit high TET1 expression following MTB-related stimulation.
–
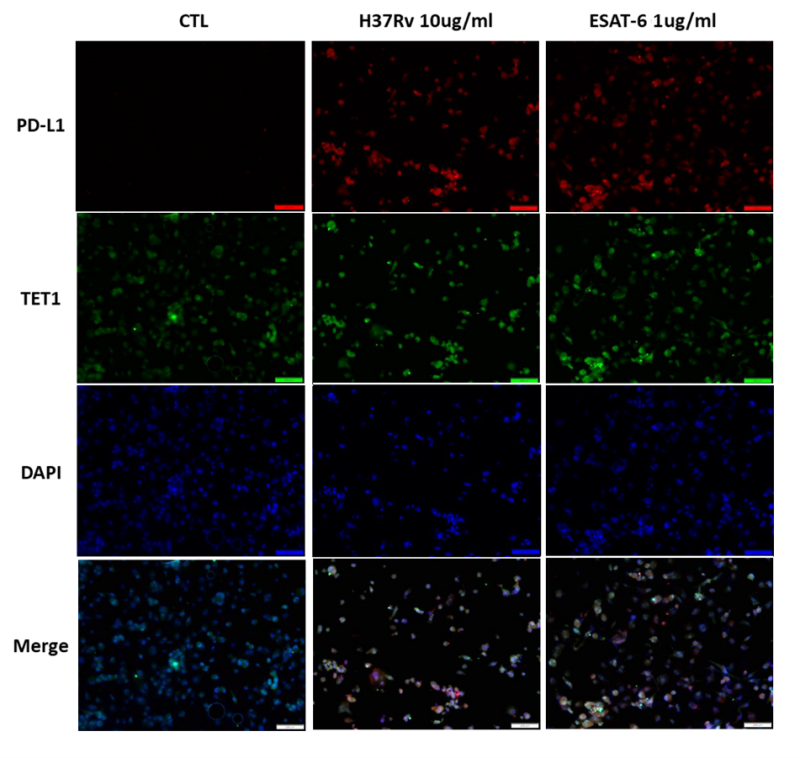
FIGURE 4: Immunofluorescence (IF) staining of THP-1 cells treated with MTB-related materials. THP-1 cells were treated with H37Rv whole cell lysate (10 µg/ml) and EsxA protein (1 µg/ml) for 24 hours. IF staining demonstrated increased expression of PD-L1 (red) overlapped with TET1 (green) in THP-1 cells. PD-L1, programmed death-ligand 1; TET1, ten eleven translocation methylcytosine dioxygenase 1.
PD-L1 production in macrophages correlated with methylation modulation
We treated THP-1 cells with MTB whole cell lysate (H37Rv, 10 and 20 μg/ml) and EsxA protein (1 and 2 μg/ml) and measured the expression of PD-L1 and methylation-related genes. Increased expression of PD-L1 and TET1, and decreased expression of DNMT1, DNMT3A, and DNMT3B, was noted in THP-1 cells after MTB-related stimulation (Supplementary Figure 1).
To elucidate how DNA methylation of the PD-L1 promoter contributes to the downregulation of PD-L1 gene expression in vivo, we employed treatment with 5’Aza to inhibit DNMT1. THP-1 cells were pre-treated with different concentrations of 5’Aza (1 μM and 5 μM), followed by treatment with MTB whole cell lysate (H37Rv, 10 μg/ml and 20 μg/ml) and EsxA protein (5 μM and 10 μM). As shown in Figure 5A, a discernible increase in PD-L1 protein expression was observed upon the treatment of THP-1 cells with MTB whole-cell lysate and EsxA protein. Notably, PD-L1 protein expression showed a further dose-dependent increase when THP-1 cells were pre-treated with 5’Aza. Consistently, PD-L1 gene expression was also increased after pre-treatment with 5’AZA in THP-1 cells (Figure 5B).
–
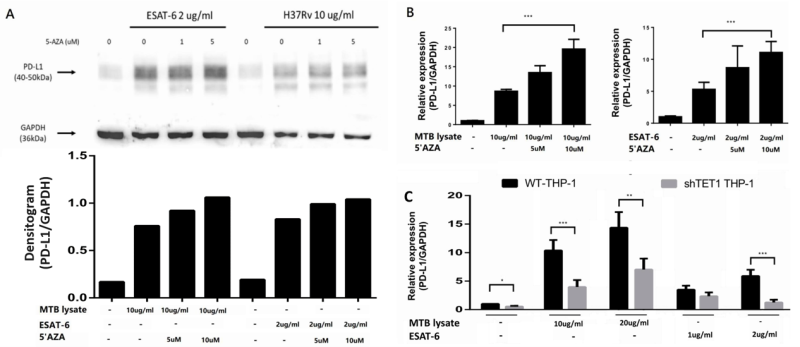
FIGURE 5: Effect of DNA methylation inhibitor and TET1 gene knockdown on PD-L1 gene expression in THP-1 cells with (A, B) pre-treatment of 5’AZA, and (C) shRNA-mediated TET1 knockdown. THP-1 cells were treated with H37Rv whole cell lysate (10 µg/ml) and EsxA protein (2 µg/ml). A) Production of PD-L1 protein was evaluated using a western blot assay with densitogram. Data are presented as the mean ± standard deviation (SD) for each group. PD-L1, programmed death-ligand 1. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
The effect of promoter methylation on PD-L1 expression was further confirmed by shRNA-mediated TET1 knockdown. PD-L1 expression was significantly decreased in THP-1 cells with TET1 knockdown, both in the presence and absence of treatment with MTB whole cell lysate and EsxA (Figure 5C). These findings provide compelling evidence for the role of promoter methylation in modulating the expression of PD-L1 in macrophages.
Decreased PD-L1 promoter methylation in active TB patients
The methylation status of the PD-L1 promoter was assessed using bisulfite sequencing, and the results are shown in Figure 6. The analysis revealed a significantly reduced methylation in the cg19724470 region, among patients diagnosed with active TB compared with that in non-TB subjects. Conversely, both active TB and non-TB subjects displayed low methylation levels in the cg14305799 region, with no statistically significant differences observed between the two groups.
–
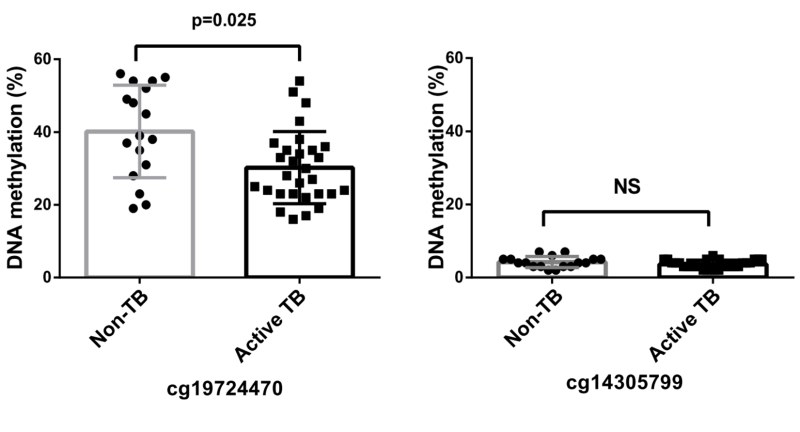
FIGURE 6: PD-L1 promoter methylation analysis in PBMCs from patients with active TB and non-TB subjects. CpG loci cg19724470 and cg14305799 were analyzed. The data in bar charts are mean values. Error bars are standard deviations. The data were compared using the Mann-Whitney U test. TB, tuberculosis.
DISCUSSIONThis prospective observational study enrolled patients with active TB and aimed to investigate the impact of DNA methylation status on PD-L1 expression during MTB-induced immune responses. Our study revealed that PD-L1 expression in PBMCs was upregulated in patients with active TB and decreased after initiating anti-TB treatment. Furthermore, we observed concurrently decreased DNMT1A expression and increased TET1 expression in patients with active TB, indicating reduced DNA methylation levels. These gene expression patterns were associated with weight loss, pulmonary involvement, and the 1-month sputum conversion status. Our subsequent in vitro experiments using human macrophage cell lines confirmed increased PD-L1 expression in macrophages upon DNMT1A inhibition and decreased PD-L1 expression upon TET1 knockdown. Finally, decreased PD-L1 promoter methylation was verified by bisulfite sequencing analysis PBMCs from patients with active TB. Taken together, our findings suggest that DNA methylation serves as one of the modulatory mechanisms that influences PD-L1 expression during MTB-induced immune responses in macrophages.
PD-1, a member of the CD28 family, is an inhibitory membrane receptor expressed on lymphocytes, while PD-L1 serves as the primary ligand for the PD-1 receptor 15, 16. Activation of the PD-1/PD-L1 pathway plays a crucial role as a negative regulator of Th1-type immunity in T cells and is considered an inhibitory factor of T cell function 17, 18. Although the role of the PD-1/PD-L1 pathway in T cell exhaustion during MTB-related pathophysiology remains controversial, it has been proposed as a potential mediator. Shen et al. demonstrated increased expression of PD-1 and PD-L1 in monocytes in both the peripheral blood and pleural fluid of patients with active TB 11. Blocking the PD-1/PD-L1 pathway was found to significantly enhance phagocytosis and intracellular killing activity of macrophages 11. Day et al. reported the upregulation of PD-1 in CD4 T cells in PBMCs from patients with smear-positive TB, and inhibition of the PD-1/PD-L1 signaling pathway augmented the production of inflammatory cytokines in CD4 T cells 19. Our previous research also indicated elevated expression of PD-L1 on monocytes and macrophages in lung tissues obtained from patients with active TB, and correlated with disease severities 13. In the present study, the expression of PD-L1 in PBMC is higher in TB patients with fever and positive sputum smear, which indicated higher bacterial burden. These findings were in line with prior studies and our previous work. It also supports the potential involvement of the PD-1/PD-L1 pathway in the pathophysiology of MTB-related immune responses.
DNA methylation is a crucial epigenetic mechanism that governs gene expression in response to external stimuli, including microorganisms. Extensive research has demonstrated the involvement of DNA methylation in regulating PD-L1 expression across various cancer types, suggesting its potential as a biomarker for predicting treatment response to immune therapies in lung cancer, prostate cancer, colon cancer, glioblastoma, and leukemia 20, 21, 22, 23, 24. Zhang et al. reported that patients with lung cancer who experienced cancer recurrence after anti-PD-1 therapy exhibited significantly higher levels of PD-L1 promoter methylation than those in the primary tumor tissues. In a mouse model, combining hypomethylation agents with anti-PD-1 therapy led to reduced tumor size 20. Gevensleben et al. demonstrated a notable inverse correlation between PD-L1 promoter methylation and mRNA expression in patients with prostate cancer. They also proposed that PD-L1 promoter methylation is a promising biomarker for risk stratification in this patient population 21. Similarly, Goltz et al. observed an inverse relationship between PD-L1 promoter methylation and PD-L1 expression in patients with acute myeloid leukemia (AML) 24. They found that higher PD-L1 promoter methylation levels were associated with a lower risk of relapse and prolonged overall survival. Collectively, these studies confirm the active involvement of PD-L1 promoter methylation in the regulation of PD-L1 mRNA expression and its impact on treatment outcomes in patients with cancer.
Despite the well-documented activation of the PD-1/PD-L1 pathway during MTB infection, the underlying mechanisms that modulate PD-L1 expression in the context of MTB pathophysiology remain poorly understood. Given the insights gained from cancer research, this study aimed to elucidate the role of DNA methylation in modulating PD-L1 expression at various levels in MTB infection. In patients with active TB, demethylation patterns were observed in several methylation-related genes, including the decreased expression of DNMT1A and increased expression of TET1. Additionally, the expression levels of DNMT1A and TET1 were correlated with the MTB burden at the time of TB diagnosis, which subsequently returned to normal levels following the initiation of anti-TB treatment. Using bisulfite sequencing analysis, we identified a specific CpG site demethylation within the PD-L1 promoter region (cg 19724470) in patients with active TB. Remarkably, an in vitro study demonstrated similar patterns of methylation-related gene expression changes in THP-1 cells treated with MTB-related stimulation. We further substantiated the impact of methylation-related genes on PD-L1 expression by inhibiting DNMTs and knocking down TET1 in THP-1 cells. To the best of our knowledge, this is the first study to explore the role of DNA methylation in modulating PD-L1 expression during MTB-related immune responses. Our findings align with previous researched in cancer, which indicated the role of promoter methylation in regulating PD-L1 expression in cancer cells 14, 21, 25. Pehrson et al. examined methylation patterns in alveolar macrophages among TB contacts, finding correlations between DNA methylation patterns and IGRA responses 26. Du et al. conducted a genome-wide methylation analysis in LTBI cases, identifying associations between methylation profiles at specific CpG loci and active TB progression 27. Additionally, a separate study observed low global methylation levels in pediatric TB patients, linking global demethylation to smear positivity 28. Together with prior literatures, our study suggests DNA methylation’s involvement in TB pathogenesis. Further research is warranted to clarify the roles of global methylation and methylation of specific promoter regions in modulating immune responses against MTB.
This study has several limitations. First, the characteristics of methylation-related gene expression were assessed in PBMCs rather than in monocytes from patients with TB. However, we could validate the coexpression of PD-L1 and TET1 in macrophages by analyzing lung tissue samples obtained from two patients with TB. Second, notable differences in demographic characteristics were observed between patients with TB and non-TB subjects, which may have contributed to variations in DNA methylation status. To address this concern, we conducted in vitro experiments to confirm our findings in patients with TB. Third, PD-L1 promoter methylation was analyzed through bisulfite sequencing of a specific promoter region rather than by employing whole-genome methylation analysis. Only two CpG sites were assessed in the PD-L1 promoter region. However, a discernible trend of demethylation was still observed at one of these sites in patients with TB. Lastly, owing to facility constraints, our in vitro studies in THP-1 cells involved treatment with MTB-related materials, including whole-cell lysates and EsxA proteins, instead of actual infection with live MTB bacilli.
In conclusion, this prospective observational study provides compelling evidence for increased PD-L1 expression in PBMCs from patients with active TB, consistent with the observed demethylation patterns in methylation-related genes. These findings were found to correlate with bacterial burden and response to anti-TB treatment. In vitro studies using human macrophages demonstrated similar patterns of PD-L1 and methylation-related gene expression upon MTB-related stimulation. Furthermore, the role of DNA methylation in the regulation of PD-L1 expression in macrophages was confirmed using DNMT inhibition and TET1 knockdown experiments. Through in vivo and in vitro evidence, our study sheds light on the modulatory mechanisms of PD-1/PD-L1 pathway activation in the innate immune responses of patients with TB. However, further investigations are warranted to explore the clinical implications and potential therapeutic strategies targeting the modulation of methylation in the management of active TB.
MATERIALS AND METHODSPatients and settings
This prospective observational study was conducted at a tertiary medical center in northern Taiwan. Eligible participants were newly diagnosed tuberculosis patients with culture-proven cases between June 2020 and July 2022. To establish a comparison group, individuals without TB were also enrolled during the same study period. Demographic characteristics, clinical presentations, and radiological presentations were collected and reviewed during the enrollment interviews. The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB number 2020-06-014BC, 2020-12-001CC). Written informed consent was obtained from each participant or their authorized representative(s) prior to enrollment. Details are shown in Supplementary material.
RNA isolation and quantitative reverse transcription PCR
PBMCs were isolated from peripheral blood samples collected from the participants on the day of enrollment. Total RNA was extracted from PBMCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Quantitative reverse transcription PCR was performed in duplicate using Power SYBR Green Master Mix (Roche, Mannheim, Germany) and analyzed using a Stratagene Mx3000P real-time PCR system. The mRNAs evaluated were PD-L1, DNMT1, DNMT3A, DNMT3B, TET1, and TET2. The relative expressions of target mRNAs were compared to GAPDH as reference. The primer sequences used for real-time PCR are provided in Supplementary Table 1.
Stimulation of human macrophages
For in vitro studies, THP-1 human leukemia cells (TIB202; ATCC, Manassas, VA, USA) were cultured in RPMI 1640 medium at a density of 1 × 106 cells/ml. The cells were differentiated into macrophages by treatment with 20 ng of phorbol 12-myristate 13-acetate (PMA, P8139; Sigma-Aldrich; St. Louis, MO, USA) for 24 h, followed by a 72-hour resting period before treatment with MTB-related stimulants. PD-L1, DNMT1, DNMT3A, DNMT3B, TET1, and TET2 expression levels were measured before and after stimulation.
MTB-related materials and in vitro stimulation
The MTB-related materials used in this study included MTB whole cell lysates (M. tuberculosis, Strain H37Rv, Whole Cell Lysate, NR-14822) and recombinant EsxA protein (Recombinant Protein Reference Standard, NR-49424; both obtained from BEI Resources; Manassas, VA, USA). THP-1 cells were treated with MTB whole-cell lysates at concentrations of 10 and 20 µg/ml and with EsxA protein at concentrations of 1 and 2 µg/mL. The treatment durations were 6 h for RNA isolation and 24 h for western blotting. PMA-treated THP-1 cells were plated in 24-well microculture plates at a density of 1 × 106 cells/well before in vitro stimulation. Details are shown in Supplementary material.
Immunohistochemistry (IHC) and immunofluorescence (IF) analysis
To identify the cells expressing PD-L1 and TET1, lung specimens obtained from two patients with active pulmonary TB were subjected to IHC analysis. Briefly, 4 µm sections of lung tissues were pretreated using an antigen retrieval solution and then incubated with anti-PD-L1 antibody (R&D Systems) and anti-TET1 antibody (GeneTex, Irvine, CA, USA).The bound antibodies were detected using a horseradish peroxidase-conjugated compact polymer system. IHC staining was also performed using an anti-CD68 antibody (Dako; Glostrup, Denmark) to identify the macrophages in lung tissues.
For IF analysis, THP-1 cells with and without MTB-related stimulation were stained using primary antibodies against PD-L1 (GeneTex; Irvine, CA, USA) and TET1 (GeneTex; Irvine, CA, USA). Nuclear staining was achieved with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images were captured using a 3D HISTECH PANNORAMIC SCAN system and were analyzed using panoramic viewer software. Details are shown in Supplementary material.
DNMTs blockade with 5’Aza and western blot assay
To investigate the role of DNMTs in PD-L1 expression, THP-1 cells were treated with the DNMTs inhibitor, 5-Aza-2’-deoxycytidine (5-Aza) (Sigma-Aldrich), at concentrations of 1 μM and 5 μM for 96 hours prior to stimulation with MTB-related materials. For western blotting, proteins harvested from THP-1 cells were electrotransferred onto polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies against PD-L1 (Cell Signaling Technology, Danvers, MA, USA) for 3 h and then with anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies for 1 h. The blots were visualized using an enhanced chemiluminescence western blot detection system. Details are shown in Supplementary material.
TET1 knockdown with shRNA
TET1 knockdown THP-1 cell lines were generated by stably expressing short hairpin RNAs (shRNAs) targeting TET1 mRNA. THP-1 cells were transduced with TET1 shRNA (TRCN0000075026) or copGFP control lentiviral particles (C6-4-2) obtained from the National RNAi Core Facility at Academia Sinica, Taiwan. The sequences of TET1 shRNAs are listed in Supplementary Table 1.
Details are shown in Supplementary material.
Promoter methylation analysis by bisulfite sequencing
CpG methylation analyses were conducted by bisulfite sequencing as previously described 29. Genomic DNA extracted from the PBMCs was treated with bisulfite. PCR was performed on bisulfite-treated DNA to amplify the promoter regions of PD-L1. Considering the previously reported high methylation status of cg19724470 and cg14305799 30, methylation-specific primers were designed to cover these two CpG loci and used for further investigation. Details are shown in Supplementary material.
Statistical analysis
Intergroup differences were analyzed using a non-parametric, two-tailed Mann-Whitney U test for numerical variables and Fisher’s exact test for categorical variables. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS version 25.0 (Chicago, IL, USA) and GraphPad Prism 7.0 (San Diego, CA, USA).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Comments (0)