The use of model organisms to understand essential processes is a well-known strategy in life science research. If we take a look at the publication statistics in repositories like PubMed, we can see that there are a substantial number of studies using different model organisms. For example, a quick search in the aforementioned repository resulted in (accessed on 2023.10.16) 142,277 matches for the keywords ‘Saccharomyces cerevisiae’, 429,254 for ‘Escherichia coli’, 1,945,515 for ‘Mus musculus’, 92,105 for ‘Arabidopsis thaliana’, 62,541 for ‘Drosophila melanogaster’, 36,775 for ‘Caenorhabditis elegans’ just a few to mention. This leads us to conclude that the contribution of model organisms to our understanding of basic biological processes is indispensable.
The yeasts have a special place among model organisms because these tiny fungal cells have provided many useful models for different studies. For example, Candida albicans and Cryptococcus neoformans emerged as models for studying fungal pathogenesis, while S. cerevisiae and Schizosaccharomyces pombe are useful models for studying the eukaryotic cell cycle and other countless fundamental biological processes. Accordingly, thousands of research articles related to these species are published every year (Fig. 1). Although S. cerevisiae is the most popular yeast model, we would like to concentrate on the fission yeasts (Schizosaccharomyces) as models in this particular review. The fission yeasts are widely established models of the eukaryotic cell cycle, cell size maintenance, cellular aging, gene expression and epigenetics, autophagy, and apoptotic processes, just a few to mention.
–
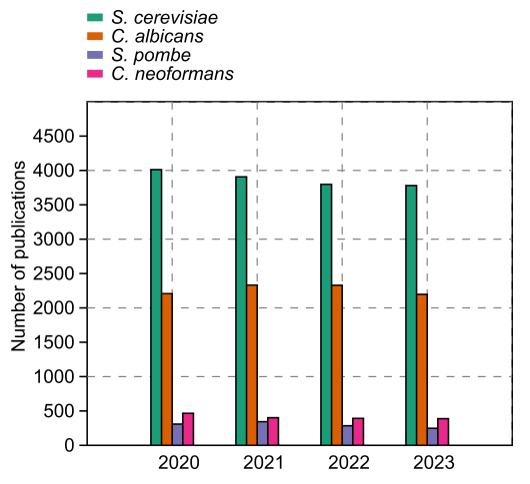
FIGURE 1: Number of published papers at the PubMed repository from 2020 to 2023. S. cerevisiae is for the baker’s yeast Saccharomyces cerevisiae, and C. albicans stands for the opportunistic human pathogen Candida albicans. C. neoformans represents the medically important species Cryptococcus neoformans, and S. pombe is for the fission yeast model Schizosaccharomyces pombe.
To our best knowledge, the fission yeast genus consists of six species to date: S. japonicus, S. pombe, S. octosporus, S. cryophilus, and the recently described species S. osmophilus and S. lindneri, and other variants 1, 2, 3, 4, 5, 6. S. japonicus has two main varieties: var. japonicus and var. versatilis, which have recently been proposed to be considered as two different lineages 7. In our opinion, the two most divergent branches of the genus (S. japonicus and S. pombe) have tremendous potential (not just) as model organisms.
FISSION YEASTS? FOR WHAT?Someone may ask the legitimate question: why do we need fission yeasts when we already have well-established and widely used yeast models such as S. cerevisiae and C. albicans? What can fission yeasts provide that cannot be provided by the aforementioned ones? Most importantly, could a fission yeast be a good (or better) alternative for studying human diseases than budding yeasts? We try to provide answers to these questions while revealing some fundamental differences among the yeast models (Table 1).
TABLE 1. Fundamental differences of yeast models.
Genome stats/
Biological features
C. albicans
S. cerevisiae
S. pombe
S. japonicus
Genome size (Mb)
~ 14.28
(SC5314)
~ 12.24
(S288c)
~ 12.59
(L972)
~ 16.6-18.12 (ATCC10660)
Chromosome number
(haploid set)
8
16
3
3
Chromosome sizes (Mb)
0.95-3.19
0.23-1.55
3.5-5.7
~ 3.8-5.75
Coding gene number
6030
5850
5134
4942
Common orthologues with humans
~ 3400
~ 3427
~ 3422
~ 3316
Disease-associated transcripts
YTBD*
~ 1000
1521
YTBD*
Genetic code
CTG
Standard
Standard
Standard
Whole Genome Duplication
pre
post
pre
pre
Preferred chromosomal state
2n
2n
1n
1n
Centromere sizes
3-4.5 kb
125 bp
35-110 kb
610-738 kb
Centromere type
Unique DNA sequence, without repetitive elements
Small, point-like
Large, repetitive sequences
Large, repetitive sequences and transposons
RNAi components
Yes
No
Yes
Yes
RNAi-mediated splicing
No
No
Yes
Yes
Percent proportion of introns
4-6%
2-6%
>50%
>50%
Spliceosome components
Yes
Reduced
Yes
Yes
Alternative splicing
Obscure
Obscure
Frequent
YTBD*
Generation time (hours)
1.7-3.6
1.25-2.0
2.0-3.0
1.0-1.5
Working time of genetic cross (days)
Not applicable
7
4
2.5
Pathogenicity
Yes
Can be
No known cases
No known cases
Hyphae production
Yes
Pseudo
No**
Yes
Cell division
Budding
Budding
Fission
Fission
Mitosis
Closed
Closed
Closed
Semi-open
DNA methylation
Yes
No
No
YTBD*
H3K9 methylation
No
No
Yes
Yes
Fundamental considerations
At first, we should take a close look at the phylogenies of the fission yeasts. Since they are a basal lineage of the Ascomycota (subdivision Taphrinomycotina), they have a closer phylogenetic relationship with the Metazoa lineage 44, 45, 10, 46, 47. Besides, the fission yeast genus has remarkably conserved common gene content, which is maintained through a relatively long divergence time 10, 48, 49. Maybe that is one of the reasons for them to preserve many common features with the higher eukaryotes. The fission yeasts are already considered “micro-mammalian” model organisms since they share various fundamental features with the metazoan species, such as chromosomal structure and metabolism, relatively large chromosomes and centromeres, low-complexity replication origins, epigenetic mechanisms for regulation of gene expression and centromere maintenance, G2/M control of cell cycle, cytokinesis, mitosis and meiosis, DNA repair and recombination, the mitochondrial translation code, spliceosome components with functional alternative splicing, post-translational modifications, and RNA interference (RNAi) 10, 11, 50, 51, 52, 53.
Chromosomes, centromeres and heterochromatin
The haplontic chromosomal state facilitates genetic modifications and makes the phenotypic association of the mutation more comprehensible. Although both S. cerevisiae and S. pombe are able to maintain haplontic and diplontic chromosomal states as well, in contrast to S. cerevisiae, the fission yeasts preferred the haplontic state. While it seems to be a tendency that the lab strains of S. cerevisiae drive towards diploidization after a few generations, the fission yeasts naturally maintain their haplontic form even in the wild 53, 20, 54, 21, 55, 56, 57. Despite possessing similar genome sizes, S. cerevisiae has many small chromosomes with short (125 bp) point-like centromeres, while the fission yeasts have few but long chromosomes with large centromeres containing repetitive sequences that are more similar to mammalian centromeres 23, 58. Nevertheless, the larger chromosome sizes allow for more efficient microscopic examination. Moreover, the fission yeast genome contains regions of centromeric heterochromatin, which is maintained by H3K9 methylation of nucleosomes and RNAi, unlike the budding yeasts that do not have the necessary molecular toolkit for either one 10, 58, 59, 26, 60. Although both S. pombe and S. cerevisiae have silent chromatin at telomeres, at the mating-type loci, and rDNA regions, only S. pombe has silent chromatin at centromeres 61, 40. In humans, the methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the folate metabolic pathway, loss of function mutations of which are associated with several human conditions, such as cancer, congenital heart disease, and maybe Down and Turner syndrome, too 62, 63, 64, 65, 66. Lim and co-workers examined the fission yeast equivalent of MTHFR, the Met11, and they revealed that it functions to maintain centromeric integrity to ensure precise chromosome segregation in mitosis and meiosis, as the Δmet11 null mutant showed increased missegregation of chromosomes in mitosis and increased transcription from centromeric heterochromatic regions 67. They also observed heterochromatic derepression at subtelomeric and rDNA regions, accompanied by a disruption of H3K9me2 and HP1 protein (Swi6) at all these loci 67. The human nucleosome remodeling and deacetylase (NuRD) complexes sustain specific gene expression programs required for lineage specification, so they have an important role in development and aging 68, 69. In many cases of cancer, the subunits of the NuRD complex contain mutations 70 and some of the mutations can also have detrimental effects on neurological and cognitive development 71. To understand the fundamental function and operation of this heterogenic complex, examination of the fission yeast counterpart Snf2/Hdac Repressive Complex (SHREC) and its interacting partners can be a good alternative 72, 73, 74, 75. Wei and co-workers studied the TOR signaling pathway, and they showed that this cascade targets a conserved nuclear RNA elimination network to dynamically control gene expression by promoting RNA decay and facultative heterochromatin assembly 76. Since RNA elimination factors are involved in proper meiotic progression during oogenesis and/or spermatogenesis in mammals, their result may shed light on the epigenetic reprogramming during development 76, 77, 78, 79. Thus, the fission yeasts proved to be a very powerful model for the investigation of heterochromatin assembly and epigenetic gene silencing 53. Surprisingly, unlike higher eukaryotes, and many other fungal species, neither S. pombe nor S. cerevisiae have DNA methylation processes 40, 41. However, the heterologous expression of a murine DNA methyltransferase in S. cerevisiae resulted in methylated DNA at specific sites 80.
Telomere maintenance
All eukaryotic organisms have precisely defined regions called telomeres at both ends of their chromosomes. Telomere malfunction can cause several problems, from genome rearrangements to several diseases like premature aging, dyskeratosis congenita, and cancer amongst many other diseases 81, 82. One of the protein complexes, the heterotrimeric CST complex plays a key role in the regulation of telomere extension, which can be examined in both the budding and the fission yeast systems 83. The other complex, which contains up to six different proteins, the shelterin-complex has a crucial role in the maintenance of telomeres, as it is responsible for telomere protection and telomerase regulation 83, 84, 85. Strikingly, S. pombe has a shelterin-like telomere complex, which lacks in S. cerevisiae 83, 84. Although the fission yeast shelterin-like complex has “only” three obvious protein orthologues with the vertebrates, the overall structure seems to be quite similar 83, 84, 86, 87, 88, 89. Thus, fundamental processes can be investigated in the fission yeasts also in the case of shelterin function 90, 91, 92. As an example, Irie and co-workers observed in S. pombe that simultaneous inactivation of the shelterin complex subunits Taz1 (TERF1 in humans) and Rap1 (TERF2IP in humans) enables a substantially higher number of gross chromosomal rearrangements per cell division, not just in the telomeric regions but also in the whole genome 93. This is also remarkable because extensive chromosomal rearrangements have been reported in many cancers with mutations in the human shelterin complex 81, 94.
Introns and splicing
Since the fission yeasts have thousands of introns in their genes compared to the few hundred introns of S. cerevisiae, and have degenerate splice site sequences and exonic splicing enhancers, the former species is again a better choice for investigating maturation of mRNA and misregulated splicing 53, 28. Although spliceosome components are available in fission yeasts, functional alternative splicing (AS) has been debated because of the low amount of unequivocal evidence. Montañés and co-workers provided exact proof for functional AS and they showed that it is more prevalent in S. pombe than it was previously thought 29. They have identified 332 alternative isoforms affecting 262 coding genes, 97 of which occur with frequencies >20%. The overwhelming majority of the events (~80%) were intron retention, besides intron inclusion, the use of alternative splicing sites, and exon skipping. According to Zheng and co-workers, the phenomenon of intron retention is one of the least understood forms of alternative splicing in the human genome, even though it can be associated with serious diseases, such as Alzheimer’s disease and cancer 95.
Protein interactions
Thanks to modern sequencing techniques, we were able to identify thousands of mutations associated with diseases and disorders in humans. However, it is still a serious problem to filter out the noise and find the true causes of the observed phenotypes. Moreover, the International Rare Disease Research Consortium (IRDiRC) also acknowledged that different model organisms are an effective experimental system for investigating the impact of gene variants on protein activity, determining their biological function, and identifying potential therapies 96. Thus, yeasts as a system seem to be good candidates for this task too 97, 98, 99. To establish binary protein-protein interactions (PPI) and to find out which mutation causes loss of function or reduced functionality, the yeast two-hybrid (Y2H) system is a well-established method 100, 101, 102. For example, a SARS-CoV2 – human protein interactome was examined in a recent study with the combined usage of Y2H and mass spectrometry 103. Yeasts can also be used for heterologous expression of other eukaryotic proteins, as well as for studying the impact of the foreign protein on the yeast transcriptome and proteome or the effect of different drugs on the proteins to be tested 104, 105, 106, 107. However, these tasks are easier when the interactome of the host is more similar to the tested one. Vo and co-workers created a proteome-wide binary interaction network for S. pombe, and they compared the result with previous data concerning the S. cerevisiae and human interactomes 108, 109, 110, 111. Interestingly, they found that only ~40% of S. pombe interactions are conserved in S. cerevisiae, but ~65% of S. pombe interactions are conserved in humans despite the overall higher sequence similarity between S. pombe and S. cerevisiae 108. Their results therefore suggest that many of the interactions between humans and S. pombe are conserved, but specifically lost in the S. cerevisiae lineage. Besides, they tested whether known disease-causing mutations that disrupt PPIs in humans also disrupt PPIs in S. pombe. Their results showed that in the three tested cases (NMNAT1-NMNAT1, PCBD1-PCBD1, and SNW1-PPIL1), the introduced mutations in the S. pombe counterparts also disrupted PPIs.
Disease-associated genes
The idea that yeast might be a useful model of human diseases has already emerged right after the completion of the sequencing of both S. cerevisiae and S. pombe 11, 12. Based on data from Heinicke et al., S. cerevisiae has approximately 1000 genes, which have orthologues in gene families associated with human diseases 17. In the case of S. pombe, we have an up-to-date and relevant information source on this topic, since the PomBase database is in connection with the Monarch Initiative 112, 113 and Mondo database 114. According to PomBase (https://www.pombase.org accessed on 2024.01.13), S. pombe has 1514 transcripts (proteins and ncRNAs) that are considered orthologues of human disease-associated transcripts 14.
THE ADVANTAGES OF FISSION YEASTS IN VIRUS RESEARCHViruses can cause various and often fatal diseases. Effective prevention and treatment of these diseases require extensive knowledge about the molecular mechanism of the infection and the changes caused by the viral proteins in the host cells. Various model organisms are used as hosts to reveal consequences of viral infections. The yeasts belong to these model organisms 115, because of their attractive features, such as eukaryotic cell structure, small genome, widely available molecular tools, and the ability of several eukaryotic viruses to replicate in their cells 20, 116, 117. That is, yeast cells are suitable for heterologous expression and the study of viral proteins.
Here, we would like to provide a brief insight into the research results of viral proteins produced in the fission yeast S. pombe, with particular attention to the Human Immunodeficiency Virus type 1, (HIV-1) Vpr protein, which has been extensively studied in this yeast species.
HIV1 causes Acquired Immunodeficiency Syndrome (AIDS) by damaging the immune system, which is a life-threatening condition. The HIV-1 genome contains several genes, and each protein encoded by these genes has a special role 118, 119. Cloning of these viral genes into S. pombe-specific vectors allowed the researchers to determine the exact cellular localization of the GFP (Green Fluorescent Protein)-tagged viral proteins in the yeast cells 119. The localization of many proteins was revealed for the first time, while further results demonstrated that the intracellular localization of the viral proteins was the same in the yeast and human cells 119.
The Vpr gene (Virus protein R) which encodes a component of virus particles that promotes virus infectivity, has been studied in detail 118. One of the goals was to find out which cellular processes of the host cells are affected by the Vpr protein and whether the same processes are inhibited in the yeast cells and the human cells. Since the S. pombe genomic sequence 14, and the genetic background of its cell processes were well-known, and in addition, a large number of mutant strains were available in this species, it was possible to express the Vpr gene both in the wild-type and various mutant strains. The overproduction of the Vpr gene product revealed that the Vpr protein caused multiple effects on the host cells. The expression of the viral protein resulted in small colonies, growth delay, abnormal cell morphology, arrest in the G2 phase of the cell cycle, and cell death 120, 121, 122, 123, 124. Besides, the Vpr protein caused depletion of the glutathione, and oxidative stress, stimulating the production of reactive oxygen species (ROS) 124, 125, 126. In addition, the direct interaction of the Vpr protein with the proteosome complex, which is responsible for ubiquitin-mediated protein degradation, has also been demonstrated (Fig. 2) 127.
–
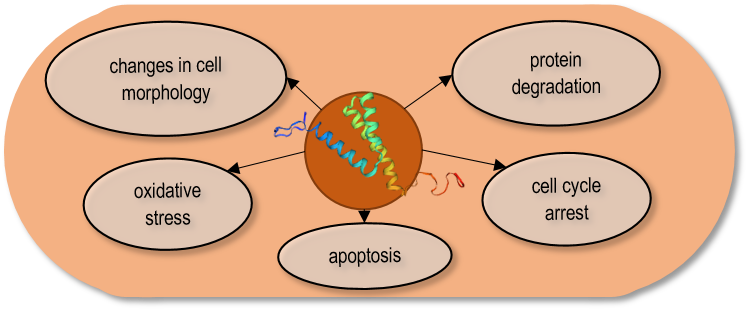
FIGURE 2: The Vpr protein caused multiple effects on the S. pombe cells.
To find out how a single viral protein can destroy various cellular processes, the phenotypic changes of the transformed yeast cells were investigated. Examination of cell morphology of the Vpr-transformed cells showed that the changes were caused by several cellular abnormalities, such as disruption of actin cytoskeleton or altered cell polarity 121. Cloning and transformation of the mutant Vpr genes enabled the detection of the effect of a given mutation on the Vpr function. The results obtained in S. pombe showed highly similar changes to the human cells that confirmed the conservation of the Vpr functions. Besides, the truncated genes also revealed that the C-terminal end of the Vpr protein was particularly important for the cell cycle (G2) arrest, while the N-terminal region was required for nuclear localization 128. Chen’s report also demonstrated that the nuclear localization of the Vpr protein was not required for G2 arrest, while it was necessary for cell killing, suggesting that the G2 arrest and cell death caused by Vpr could be independent functions 128.
The investigation of the Vpr-expressing yeast cells shed also light on the molecular background of the cell cycle arrest. The experiments proved that cell cycle arrest correlated well with increased phosphorylation of the Cdc2 kinase, which is the key regulator of mitosis 120, 129. These experiments showed that the regulators of the Cdc2, such as wee1 (encodes an M-phase inhibitor protein kinase) and the cdc25 (encodes a phosphatase, M-phase inducer) were important in the Vpr-induced cell cycle arrest 130, 131. According to the data, the Vpr protein promoted the cytoplasmic compartmentalization of Cdc25 and inhibited its function, which required the Srk1 kinase 123. Since there are differences in cell cycle regulation between S. pombe and S. cerevisiae (the G2/M transition is more important in S. pombe than S. cerevisiae) 53, it was better to choose the fission yeast for the analysis of Vpr-mitosis relation.
The further results also showed that the Vpr protein might affect the cell cycle through different pathways because the rad24 gene (which plays a role in the DNA damage pathway) was also involved in the Vpr-associated cell division defect
Comments (0)