Hailey–Hailey disease (HHD) or familial benign chronic pemphigus, an uncommon, autosomal dominant genodermatosis with complete penetrance is characterised by flaccid vesicles and blisters, with a predilection for intertriginous regions, such as the retroauricular folds, axillae, lateral aspects of the neck, and inguinal and perianal regions.1 A genetic defect in the Adenosine Triphosphatase (ATP) calcium-transporting type 2C member 1 gene (ATP2C1) located on chromosome 3q21-q24 causes this disease.2ATP2C1 encodes a protein named human secretory pathway Ca2+/Mn2+-ATPase pump type 1 (SPCA1), which is present in the Golgi apparatus membrane and plays a role in Ca2+/Mn2+ transportation, maintaining a balance between Ca2+ concentration in the cytoplasm and Golgi apparatus.3 A hereditary mutation in ATP2C1 results in insufficient SPCA1 levels, leading to a reduction in Ca2+/Mn2+ concentrations in the Golgi apparatus. This alteration disrupts the functions of Golgi apparatus proteins that depend on normal Ca2+ levels, causing abnormalities in intercellular connections in keratinocytes and ultimately leading to desquamation in the skin epidermis.
Despite knowing that ATP2C1 mutations are the primary cause of HHD, the precise molecular mechanism of the gene in HHD has not been elucidated. The widespread desquamation of spinous-layer cells in HHD is probably associated with the levels of tight junction proteins and desmosomes. In a study, an in vitro cell model was established by ATP2C1 transfection, and its knockdown showed that the levels of desmosomes remained unchanged. In contrast, the levels of the tight junction protein claudin increased, and those of zonula occludens protein 1 (ZO-1) decreased.4 Therefore, we shifted our focus from desmosomes to exploring the link between HHD and tight junction proteins. ZO-1 directly interacts with occludin, claudin 1, and claudin 4 via the post-synaptic density disc large Zo-1 domains. In contrast, its C-terminus directly binds to F-actin to interact with the F-actin cytoskeleton.5,6 The actin-severing protein cofilin binds to the phosphorylated region of SPCA1. It gets phosphorylated to form P-cofilin, which, in turn, interacts with F-actin, resulting in SPCA1 activation and Ca2+ and Mn2+ pumping into the Golgi apparatus lumen under adequate ATP supply.7 Moreover, epithelial cells participate in cell adhesion and tight junction protein connections with the help of Ca2+ and F-actin polymerisation.8 Therefore, we hypothesised an association between HHD occurrence and P-cofilin and F-actin levels.
Moreover, we performed this study to evaluate the relationship between the levels of SPCA1 and cytoskeletal or tight junction proteins in HHD skin lesions. Furthermore, the effect of ATP2C1 knockdown on these protein levels and morphological acantholysis was analysed using keratinocytes.
Materials and Methods Human tissue samplesHHD skin samples were obtained from patients (n = 22) with HHD after examining their clinical features and histopathology. Normal skin tissues were collected from healthy donors (n = 20) undergoing plastic surgery. For keratinocyte experiments, human foreskin tissues were isolated from healthy patients who underwent circumcision. This study was performed under the guidance and supervision of the Hospital Research Ethics Committee, and written informed consent was obtained from all subjects.
Immunohistochemistry (IHC)As described previously,9 tissues were cut into 5-mm paraffin sections. The sections were subjected to dewaxing treatment, followed by antigen retrieval with sodium citrate. After incubation with 2% goat serum (Beyotime Biotech, Shanghai, China), the samples were incubated with primary antibodies at 4℃ overnight. These primary antibodies were mouse antibodies against SPCA1 (Proteintech, Rosemont, Illinois, USA), F-actin (Abcam, Cambridge, MA, USA) and rabbit antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Proteintech), occludin (Abcam), claudin 1 (Abcam), claudin 4 (Abcam), ZO-1 (Abcam), and P-cofilin (Cell Signaling Technology, Danvers, MA, USA). Then, the sections were incubated with a horseradish peroxidase-labelled goat anti-Rabbit/Mouse antibody (DAKO, Glostrup, Denmark) and the 3, 3’-diaminobenzidine chromogen substrate (DAKO). Phosphate-buffered saline controls were used for all samples.
Cell culture and short hairpin RNA (shRNA) transfectionKeratinocytes were obtained as described previously,10 and cultured in the keratinocyte serum-free medium (KFSM) with low Ca2+ (0.09 mM; KSFM; Sciencell, San Diego, CA, USA). The cultured cells at passage 2 or 3 were divided into blank, control shRNA, and ATP2C1 shRNA lentivirus transfection groups and seeded on 6-well plates. A total of 20 µl of shRNA lentivirus (5×10 7 TU/mL) and enhancement solutions (Genechem, Shanghai, China) were added to the culture medium of the ATP2C1 shRNA group. The exact amounts of the harmful virus and enhancement solution were added to the control shRNA group. After 8 h of transfection, the medium was replaced with regular KSFM. After culturing for 72 h, the keratinocytes were further cultured in the KSFM containing 1 µg/mL puromycin for cell selection. When the blank-group cells were completely killed by puromycin, and no more cells died in the ATP2C1 shRNA group, the surviving cells were used for further experiments.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)Total RNA was extracted from fresh tissues and cell cultures using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was reverse transcribed into complementary DNA (cDNA) using a commercial cDNA kit (Takara Bio, Shiga, Japan). Then, qPCR was performed on a 7900HT Fast PCR System (Applied Biosystems, Waltham, MA, USA). SYBR Green Master Mix (Takara Bio, Shiga, Japan) was a fluorescent dye. The synthesis of all target gene primers was performed by Bioscience (Shanghai, China) using GAPDH primers as internal references. The primer sequences are listed in Supplementary Table 1.
Cellular immunofluorescenceThe cells were cultured in glass-bottom culture dishes (MatTek, Ashland, MA, USA) and fixed with the 4% paraformaldehyde (PFA) solution. The cells were permeabilised using Triton and subsequently blocked using a goat serum. Then, the cells were incubated with primary antibodies (see above under (IHC)) at 4°C overnight. Subsequently, AL488-labelled goat anti-rabbit/mouse IgG (Beyotime, Shanghai, China) was added for staining. After incubating with the 4¢,6-diamidino-2-phenylindole (DAPI) solution (Het Biotech, Xi’an, China), the cells were observed under a digital confocal microscope (Leica, Wetzlar, Germany).
Western blotting techniqueProtein lysates were prepared from the cell cultures using the radioimmunoprecipitation buffer (Het Biotech). Protein concentrations were determined using the Bicinchoninic Acid Protein Assay Kit (Het Biotech). The samples were separated on electrophoresis gels, followed by transfer onto polyvinylidene difluoride membranes (Millipore, Burlington, MA, USA). Horseradish peroxidase goat anti-rabbit/mouse immunoglobulin G (1 µg/ml; Abcam) was used as a secondary antibody. Then, an electrochemiluminescence kit (Millipore) was used for signal detection. The intensities of all protein bands were compared with the intensities of Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) bands of the same filter. The obtained band intensities were quantified using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Transmission electron microscopy (TEM)The cells were fixed using the electron-microscopy-specific fixative, followed by neutral phosphate buffer-osmic acid addition. After dehydration and embedding, the cells were cut into 60–80 nm ultra-thin sections. Following double staining with 1% uranyl acetate and lead citrate, the sections were visualised, and images were captured with a transmission electron microscope (HT7700, HITACHI, Tokyo, Japan).
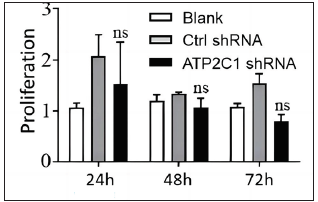
Cell proliferation and apoptosisThe cells were cultured in 96-well plates and transfected with shRNA. After transfecting for 24, 48, and 72 h, the cultures were analysed for cell proliferation. The cell counting kit-8 (CCK-8) solution (Beyotime) was added, and the cells were incubated at 37°C for 2 h. Absorbance was measured at 450 nm.
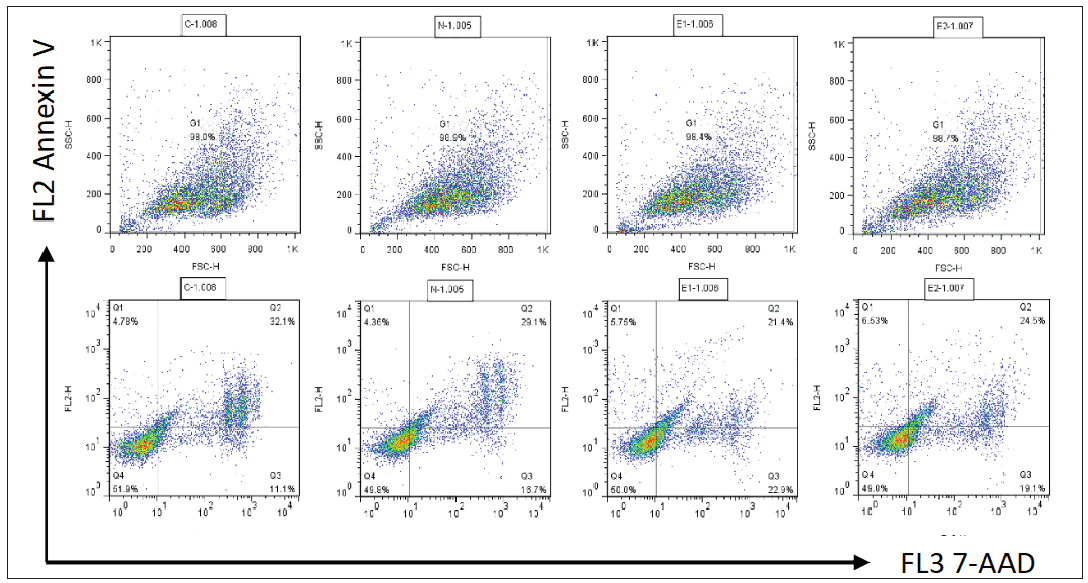
Cell apoptosis was determined using flow cytometry. After 48 h of transfection, the cells were diluted to 1 × 106 cells/mL, incubated with a binding buffer, and mixed with 7-amino actinomycin D (7-AAD) (red fluorescence) and annexin V-phycoerythrin (orange-red fluorescence). The data were analysed using the LSRII instrument (BD Biosciences, San Jose, CA, USA) and processed using the FlowJo7.6.1 software (BD, San Diego, CA, USA).
Intracellular Ca2+ detectionThe cells in the three groups were transferred to black 96-well plates and cultured for 24 h. The culture medium was then replaced with an assay buffer containing fluo-3 AM (0.5 µM, Beyotime), and the cells were further incubated at 37°C for 60 min for fluorescence probe loading.11 Intracellular fluo-3 fluorescence was then monitored at an excitation wavelength of 506 nm and an emission wavelength of 526 nm using a fluorescent enzyme labelling instrument (Labsystems Multiskan MS, Finland). Relative fluorescence values compared the intracellular Ca2+AQ concentration of the three groups.
Statistical analysisThe experimental results were subjected to statistical analysis and graphing using the ImageJ and GraphPad Prism 8.0 software. A two-sample independent t-test was performed for comparing any two groups, and an analysis of variance was performed for comparing the three groups. Differences were considered statistically significant at p < 0.05.
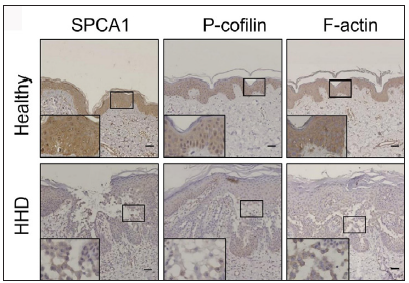
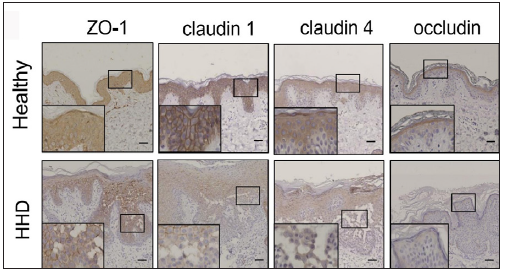
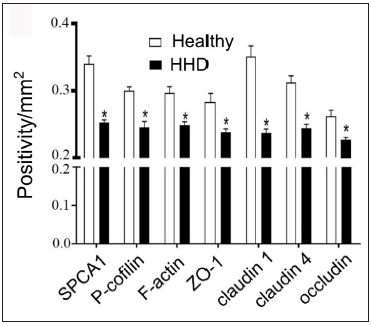
Results Cytoskeletal and tight junction proteins were deregulated in the skin lesions of HHDThe IHC analysis showed that the levels of SPCA1, F-actin, P-cofilin, claudin 1, claudin 4, occludin, and ZO-1 were high in the normal epidermis and low in the HHD lesions [Figures 1a and 1b]. Their quantitative analysis using ImageJ showed decreased levels of these proteins [Figure 1c].
Export to PPT
Export to PPT
Export to PPT
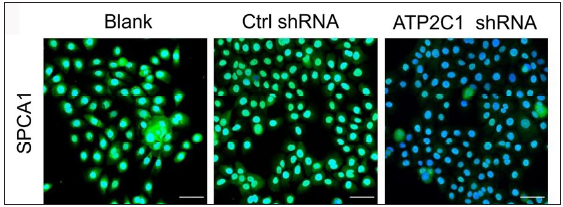
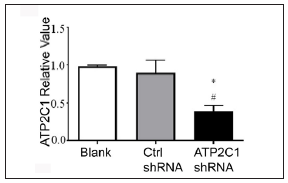
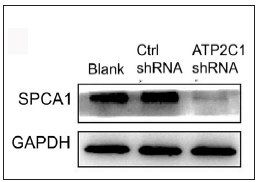
ATP21C was inhibited in cultured keratinocytes by shRNA transfectionThe keratinocytes exhibited morphological changes after shRNA transfection and returned to normal 96 after transfection, suggesting successful transfection (Supplementary Figure 1). The immunofluorescence analysis showed that SPCA1 levels decreased significantly in the ATP2C1 shRNA group with no difference between the two controls (p < 0.050) [Figure 2a]. Conformably, ATP2C1 mRNA and SPCA1 levels decreased in the ATP2C1 shRNA group [Figures 2b and 2c].
Export to PPT
Export to PPT
Export to PPT
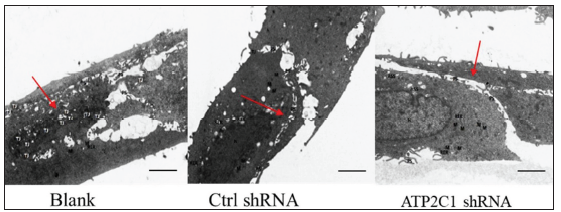
The transmission electron microscopy (TEM) results showed that the cells in the blank group exhibited no swelling. Several tight junctions were observed between the cells with narrow intercellular space. The cells in the control shRNA group showed mild swelling, with lesser intercellular tight junctions accompanied by more expansive intercellular space. The shRNA-transfected cells showed swelling with a few tight junctions and widened intercellular space [Figure 2d].
Export to PPT
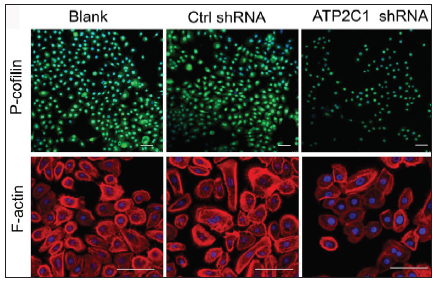
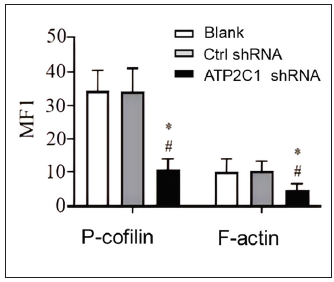
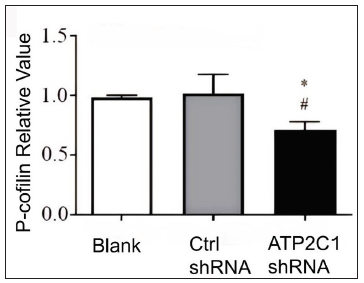
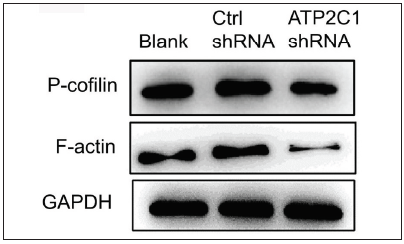
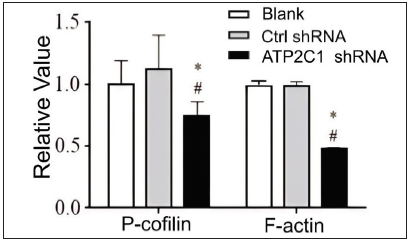
ATP2C1 knockdown suppressed P-cofilin and F-actinWe examined the levels of P-cofilin and F-actin in the cells using immunofluorescence. Compared with the blank group, the control shRNA group showed minimal changes in P-cofilin and F-actin levels. However, the shRNA-transfected cells showed significantly reduced fluorescence intensity (p < 0.05), accompanied by considerably decreased F-actin ring formation around the cell periphery and reduced F-actin filamentous pseudopodia between the cells [Figures 3a and 3b]. Compared with the blank and control shRNA groups, the ATP2C1 shRNA group showed lower P-cofilin mRNA transcript levels (p < 0.050) [Figure 3c]. Consistently, the western blotting technique results showed decreased P-cofilin and F-actin levels after shRNA transfection. (p < 0.05) [Figures 3d and 3e].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
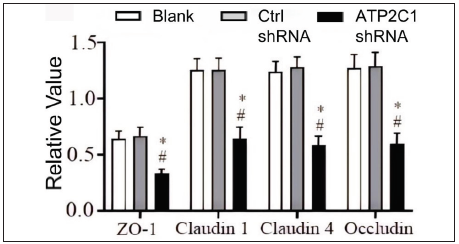
The levels of tight junction proteins decreased after ATP2C1 knockdownWe detected the levels of claudin 1, claudin 4, occludin, and ZO-1 by immunofluorescence and simultaneously performed F-actin co-staining. The fluorescent intensities of F-actin and the four tight junction proteins in the ATP2C1 shRNA group were significantly lower (p < 0.05), and no significant difference was observed between the two control groups [Figures 4a and 4b].
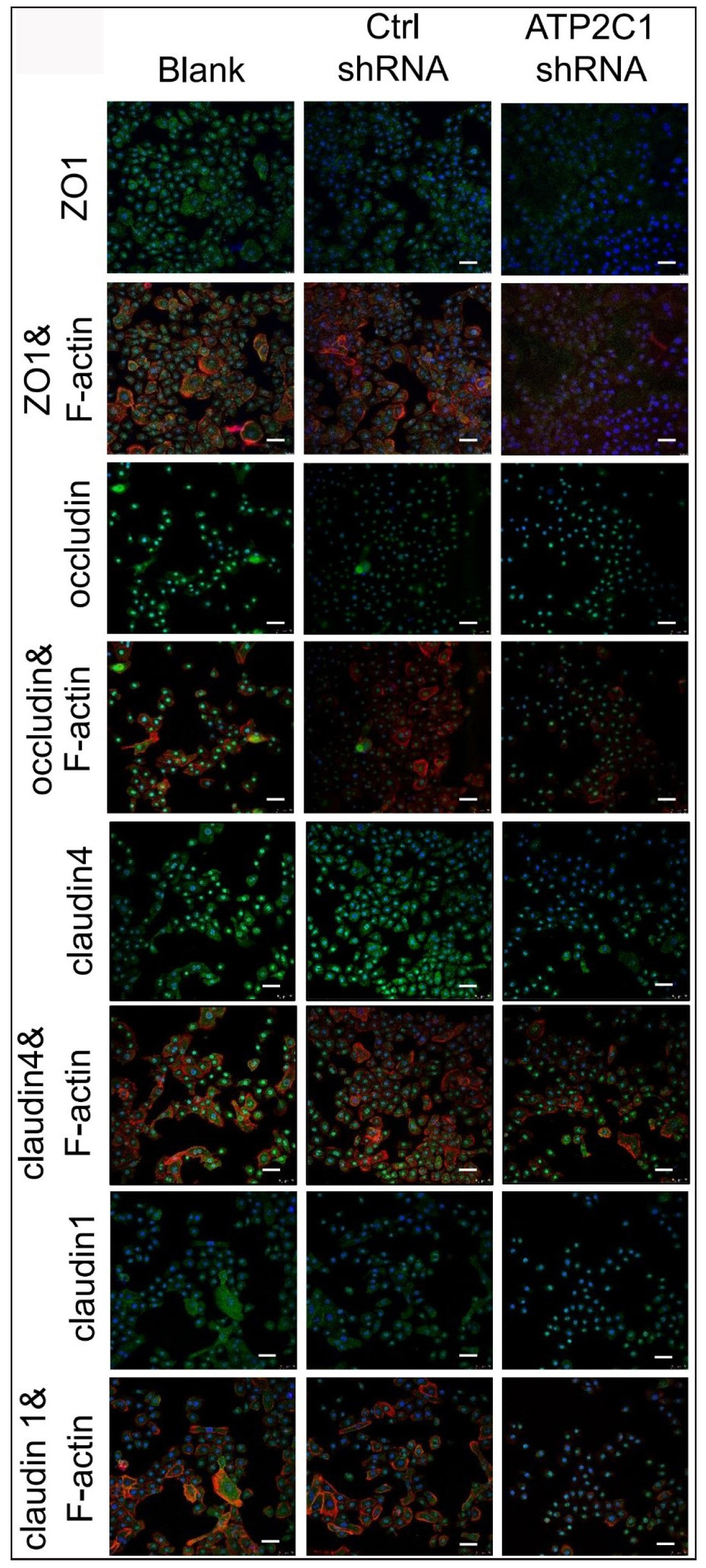
Export to PPT
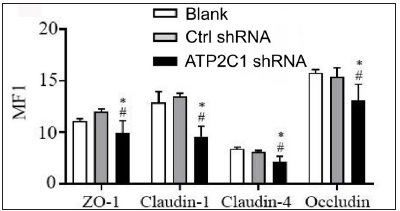
Export to PPT
Further, we analysed the mRNA and protein levels of the tight junction proteins. The mRNA levels of claudin 1, claudin 4, occludin, and ZO-1 in the ATP2C1 shRNA group decreased significantly compared with those in the two control groups (p < 0.05). [Figure 4c]. The western blotting technique results showed that the levels of the four tight junction proteins were lower in the ATP2C1 shRNA group (p < 0.05), and no significant differences were observed between the two control groups [Figures 4d and 4e].
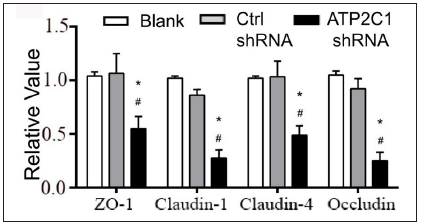
Export to PPT
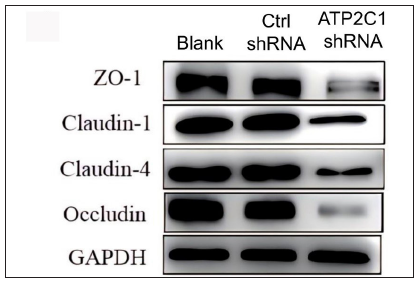
Export to PPT
Export to PPT
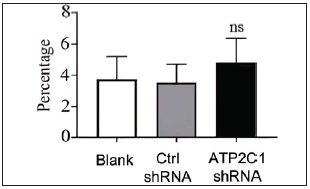
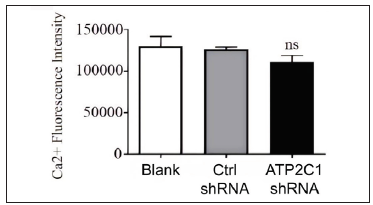
Cell apoptosis and intracellular Ca2+ influx were not affected by ATP2C1 knockdownThe flow cytometry results showed that apoptosis ratios were similar between the three groups [Figures 5a and 5b]. The CCK-8 analysis showed that cell proliferation in the ATP2C1 shRNA group was not altered [Figure 5c]. We detected intracellular Ca2+ concentrations in the keratinocytes using fluorescent microplates. However, no difference was observed in intracellular Ca2+ concentrations among the three groups [Figure 5d].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
DiscussionThe present study showed the dysregulation of cytoskeletal and tight junction proteins in HHD skin lesions. Moreover, a stable in vitro cell model of epidermal acantholysis with ATP2C1 knockdown was successfully established in human keratinocytes. The levels of the cytoskeletal and tight junction proteins decreased because of a decrease in SPCA1 expression. Furthermore, cell apoptosis and proliferation, as well as intracellular Ca2+ influx, remained unaffected after ATP2C1 knockdown.
A study showed that SPCA1 stabilises the actin cytoskeleton by interacting with cofilin, which is bound to the phosphorylated region of SPCA1 in HeLa cells.7ATP2C1 controlled mouse neural tube closure by regulating cytoskeletal dynamics.12ATP2C1 deficiency increased claudin and occludin levels in keratinocytes and decreased ZO-1 levels.4 We examined the tight junction proteins in the lesion tissues and keratinocytes (in vitro model). SPCA1 maintains a balance between the levels of cytoskeletal and tight junction proteins,4 and the present findings are consistent with this observation. Conversely, the present study showed that P-cofilin and F-actin levels decreased in the skin lesions of patients with HHD. Moreover, their levels also fell in the cultured keratinocytes after a decrease in SPCA1 expression. Thus, these results support the function of SPCA1 in regulating structural proteins related to epidermal acantholysis.
Currently, no suitable HHD animal model explicitly targets ATP2C1 mutations.13,14 Different cell models for acantholysis exhibit poor efficiency and low stability for gene downregulation.15 A yeast-based HHD model has limitations in mimicking in vivo conditions.16 Thus, to better simulate HHD, we performed shRNA lentiviral transfection in human primary keratinocytes. The results showed that both the mRNA level of ATP2C1 and the protein level of SPCA1 decreased significantly in the shRNA-transfected keratinocytes, and this characteristic was maintained even after multiple passages. Thus, we could successfully establish a cell model of HHD-like acantholysis with higher and more stable transfection efficiencies.
Cofilin binds to SPCA1 at the trans-Golgi ne
Comments (0)