Urinary tract infections (UTIs) rank among the most prevalent infections acquired both in healthcare settings and within communities, with significant implications for patient health (Shahbazi et al., 2018; Shahkolahi et al., 2022). In 2019, more than 404.6 million individuals worldwide were diagnosed with a UTI, contributing to over 200,000 deaths globally (Codelia-Anjum et al., 2023; Shivaee and Mirshekar, 2019). UTIs are particularly common in vulnerable populations, such as pregnant women, the elderly, and sexually active individuals, who are prone to both community-acquired and healthcare-associated UTIs (CAUTIs and HAIs) (Shivaee and Mirshekar, 2019; Govindarajan and Kandaswamy, 2022). While Escherichia coli strains account for 80 to 90% of UTI cases, the rising incidence of Enterococcus faecalis strains in up to 20% of cases has garnered considerable attention (Taati Moghadam et al., 2021; Abdullah et al., 2023; Shahbazi et al., 2023). In Tehran, Iran, recent studies underscore the growing concern regarding UTIs. Research conducted at local hospitals has highlighted the high prevalence of E. faecalis among UTI patients, with significant resistance to commonly used antibiotics (Minaeian et al., 2020; Dadashi et al., 2021; Samani et al., 2021; Ma et al., 2021).
The emergence of E. faecalis as a prominent UTI pathogen is alarming, particularly due to its intrinsic resistance to a broad range of antibiotics, including aminoglycosides, cephalosporins, trimethoprim-sulfamethoxazole, and macrolides (Wojnicz et al., 2016). Furthermore, E. faecalis can acquire resistance to clinically relevant antibiotics such as vancomycin, linezolid, and kanamycin, complicating treatment options (Govindarajan et al., 2022). Recent studies have highlighted a troubling increase in linezolid-resistant E. faecalis strains, with mechanisms of resistance linked to genes such as erm(A) and optrA, and mutations like G2576U in the 23S rRNA (Ma et al., 2021; Yang et al., 2024). This trend is corroborated by research in China, which reported a 22.61% prevalence of linezolid-resistant E. faecalis isolates, primarily associated with the presence of the erm(A) gene and risk factors such as indwelling catheters (Ma et al., 2021). Similarly, a study in India identified high rates of optrA gene-mediated resistance among E. faecium strains, illustrating the widespread nature of this issue (Rani et al., 2023).
The ability of E. faecalis to form biofilms, particularly in catheter-associated urinary tract infections (CAUTIs), further exacerbates its antibiotic resistance (Govindarajan and Kandaswamy, 2022). Biofilm formation is a key virulence mechanism, allowing the bacteria to evade host immune responses and enhancing their survival in harsh conditions (Rahimzadeh et al., 2023; Șchiopu et al., 2023). Recent research highlights the crucial role of virulence factors in E. faecalis infections. These include secreted factors like cytolysin (cylA), gelatinase (gelE), and hyaluronidase (hyl), as well as cell surface proteins such as aggregation substances (asa1), enterococcal surface protein (esp), endocarditis antigen (efaA), and collagen-binding protein (ace) (Aung et al., 2023; Comerlato et al., 2013; Zhang et al., 2017). A critical enzyme involved in anchoring many of these surface proteins is sortase. Sortase plays a pivotal role in the assembly of pili, which are essential for bacterial adhesion and biofilm formation. By recognizing a cell-wall sorting (CWS) motif, sortase cleaves and anchors surface proteins to the cell wall, contributing to bacterial virulence (Sivaramalingam et al., 2024). Sortase and its associated pili assembly are attractive targets for antimicrobial interventions due to their vital role in infection and biofilm development (Sivaramalingam et al., 2024).
Moreover, biofilms formed by E. faecalis often involve interactions with other species, such as E. coli. This dual-species biofilm formation enhances virulence and antibiotic resistance, driven in part by mechanisms like iron metabolism. E. faecalis biofilms have been shown to increase iron uptake via ferrous iron transporter proteins, which promotes the survival of both E. faecalis and E. coli under iron-supplemented conditions, enhancing biofilm resilience and antibiotic resistance (Govindarajan and Kandaswamy, 2022). This symbiotic relationship complicates treatment, as biofilms provide a protective environment that shields bacteria from both the immune system and antibiotics (Govindarajan and Kandaswamy, 2022).
Considering the growing clinical significance of E. faecalis, the aim of this study is to explore key attributes, including antibiotic resistance, virulence factors, biofilm formation capacity, and molecular typing through multilocus sequence typing (MLST). Understanding these factors will contribute to the development of more effective therapeutic strategies for combating E. faecalis infections.
2 Materials and methods 2.1 Bacterial isolatesBetween March 2021 and April 2022, a total of 300 non-duplicated urine samples were collected from inpatients at Shariati Hospital, Tehran, Iran. These patients were suspected of having a UTI based on clinical symptoms evaluated by healthcare professionals. The cohort included 180 males and 120 females. Inclusion criteria required that patients had not taken antibiotics within 48 h prior to sample collection, exhibited bacterial counts of ≥105 colony-forming units (CFU), and demonstrated at least one UTI symptom, such as fever, increased urinary frequency, painful urination, lower abdominal tenderness, bladder congestion, or hematuria (Shahbazi et al., 2018; Shivaee and Mirshekar, 2019; Komala and Kumar, 2013).
Midstream urine samples were collected aseptically in sterile containers and promptly transported to the clinical microbiology laboratory for examination and culture analysis. Urine samples were inoculated onto blood agar plates using calibrated loops and incubated at 37°C for 24 h. Colony morphology and phenotypic characteristics were visually assessed, and E. faecalis identification was performed using standard biochemical tests, including Gram staining, catalase testing, bile esculin hydrolysis, growth in 6.5% sodium chloride, and arabinose fermentation. Confirmation of E. faecalis isolates was achieved using a polymerase chain reaction (PCR) assay with specific primers (Table 1) (Shahroodian et al., 2022). All confirmed isolates were preserved in brain-heart infusion (BHI) broth (Merck, England) supplemented with 20% glycerol and stored at −80°C for long-term preservation.
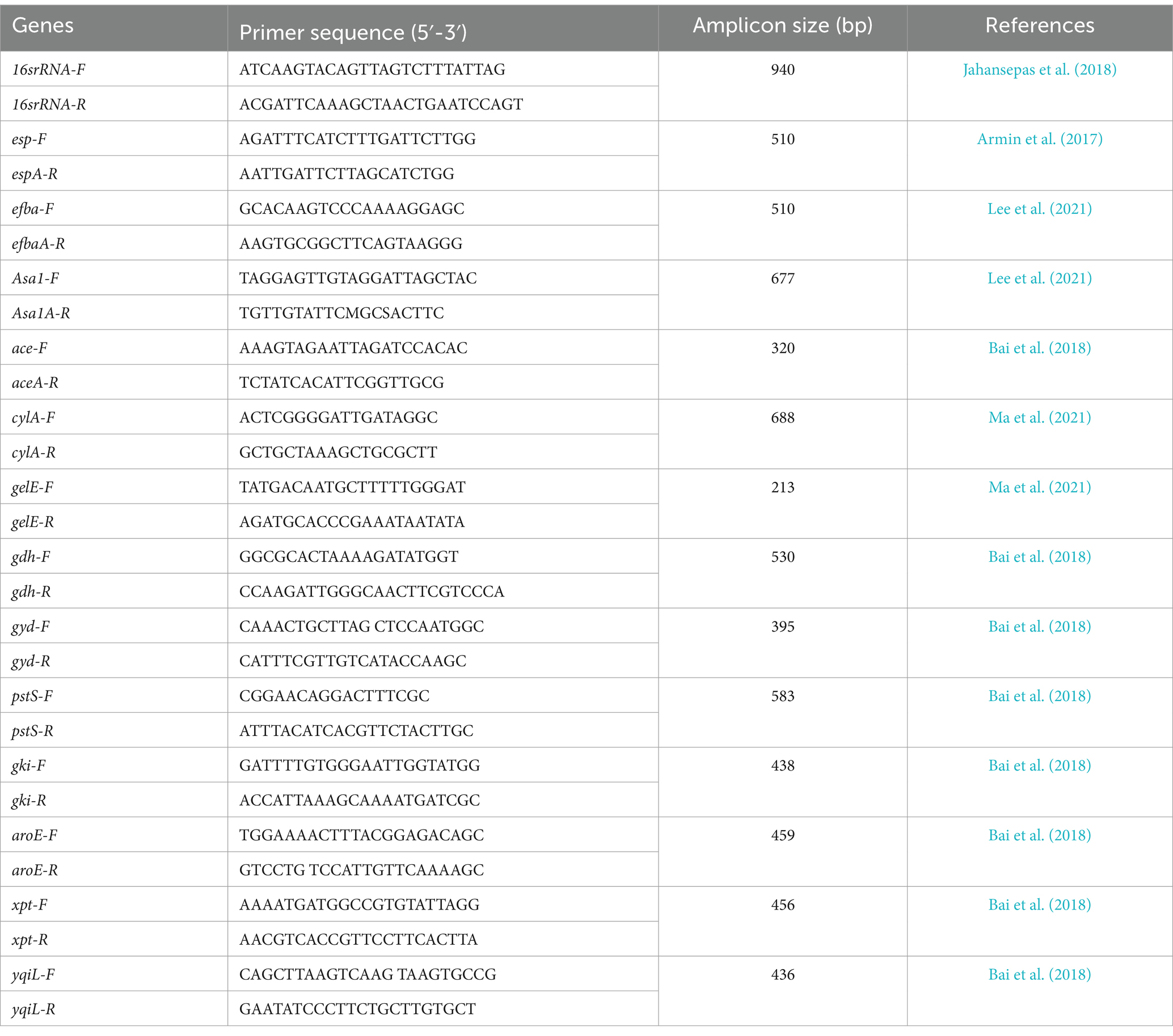
Table 1. Oligonucleotide primers used in this study.
2.2 Antibiotic susceptibility testingSusceptibility of the isolates to a panel of antibiotics was assessed using the Kirby-Bauer disk diffusion test (Traub et al., 1998). The antibiotics tested included vancomycin (30 μg), penicillin G (10 μg), ampicillin (10 μg), tetracycline (30 μg), minocycline (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), gatifloxacin (5 μg), gentamicin (120 μg), nitrofurantoin (300 μg), and linezolid (30 μg), all sourced from Mast Group Ltd., United Kingdom. Minocycline was included because, according to CLSI 2022 and 2023 guidelines, although organisms susceptible to tetracycline are typically susceptible to minocycline, some strains that exhibit intermediate or resistant profiles to tetracycline may still be susceptible to minocycline (Lewis and James, 2022). The results were interpreted in accordance with Clinical and Laboratory Standards Institute guidelines (Lewis and James, 2022), and E. faecalis ATCC 29212 served as a reference strain for comparative analysis (Khalil et al., 2022).
2.3 Virulence gene identificationBacterial DNA was extracted using the High Pure PCR Template Preparation Kit (Roche, Germany). The extracted DNA was used as the template for PCR amplification. The presence of virulence factors in E. faecalis isolates was examined, targeting key genes such as enterococcal surface protein (esp), secreted factors like cytolysin (cyl), aggregation substances (asa1), endocarditis antigen (efaA), collagen-binding protein (ace), and gelatinase (gelE) genes. The PCR conditions followed established protocols (Karimi et al., 2018), and amplification products were sequenced using an ABI 3730X capillary sequencer (Macrogen, Korea). E. faecalis ATCC 29212 served as the reference strain.
2.4 Biofilm formation assayBiofilm formation was quantitatively assessed using the microtiter plate method as outlined in previous studies (Bostanghadiri et al., 2019). Bacterial isolates were cultured in LB broth (Merck) and incubated overnight at 37°C. The cultures were subsequently diluted 1:40 in fresh TSB, and 200 μL of the diluted solution was transferred to the wells of a flat-bottomed polystyrene microtiter plate. The plate was then incubated at 37°C for 48 h. Wells containing only TSB served as negative controls. Following incubation, the plates were gently washed three times with phosphate-buffered saline (PBS; pH 7.2) to remove non-adherent cells. The wells were then fixed with 200 μL of methanol (99.8%, Sigma-Aldrich) for 15 min and allowed to air dry at room temperature. Subsequently, the biofilms were stained with 200 μL of crystal violet (1%, Sigma-Aldrich). Excess dye was removed by washing the wells three times with PBS. The crystal violet bound to the adherent cells was solubilized with 200 μL of acetic acid (33%, Sigma-Aldrich) per well. The amount of biofilm formation was determined by measuring the absorbance at 490 nm (OD 490) using an ELISA reader. Isolates were categorized based on the following criteria: strong-biofilm producers (OD > 4 × OD control), moderate-biofilm producers (2 × OD control < OD ≤ 4 × OD control), weak-biofilm producers (OD control < OD ≤ 2 × OD control), and non-biofilm producers (OD ≤ OD control) (Bostanghadiri et al., 2019). E. faecalis ATCC 29212 was used as a negative control, and all biofilm assays were performed in triplicate.
2.5 Multi-locus sequence typingMLST of E. faecalis isolates followed established methodology (Zheng et al., 2018). Internal regions of seven housekeeping genes— gyd (glyceraldehyde-3-phosphate dehydrogenase), gdh (glucose-6-phosphate dehydrogenase), pstS (phosphate ATP binding cassette transporter), yqiL (acetyl-coenzyme A acetyltransferase), xpt (shikimate 5-dehydrogenase), gki (putative glucokinase), and, aroE (shikimate 5-dehydrogenase)—were amplified using PCR. Primer sequences and references are detailed in Table 1. PCR reactions included 12.5 μL of 2X PCR Master Mix (Ampliqon, Denmark), 1 μL of each forward and reverse primer, 1 μL of DNA, and 9.5 μL of distilled water. The PCR program consisted of initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 60 s, with a final extension at 72°C for 10 min. Sequences were assigned unique allele numbers based on the E. faecalis MLST database, and the allelic profile for each isolate was generated by merging the allelic sequences from the seven genes (Zheng et al., 2018).
2.6 Statistical analysisStatistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, United States). Fisher’s exact test and chi-squared test (χ2) were used to evaluate the correlation between biofilm formation, antibiotic resistance, and virulence gene distribution. A p-value of <0.05 was considered statistically significant.
3 Results 3.1 Patient demographics and bacterial isolatesIn a cohort of 300 individuals diagnosed with urinary tract infections (UTIs), urine samples were analyzed, and microbiological testing identified E. faecalis as the causative agent in 160 cases. Consequently, the prevalence of E. faecalis in the studied UTI cases was determined to be 53%. Among these 160 E. faecalis isolates, 81 were obtained from male patients and 79 from female patients, yielding a male-to-female ratio of 1.02. The majority of the patients, specifically 45 out of 160, were within the age range of 59 to 68 years.
3.2 Antibiotic susceptibility pattern of isolatesAccording to the CLSI interpretation criteria, a substantial proportion of the isolates exhibited resistance to tetracycline (83.8%, 134/160) and minocycline (82.5%, 132/160). Conversely, resistance to vancomycin, penicillin G, ampicillin, nitrofurantoin, and linezolid was observed at notably low levels, with prevalence rates of 1.2% (2/160). Resistance to fluoroquinolones was recorded at 16.2% (26/160) for ciprofloxacin, 15.0% (24/160) for levofloxacin, and 13.8% (22/160) for gatifloxacin. Additionally, high-level gentamicin resistance was noted in 18.8% (30/160) of the isolates (Figure 1).
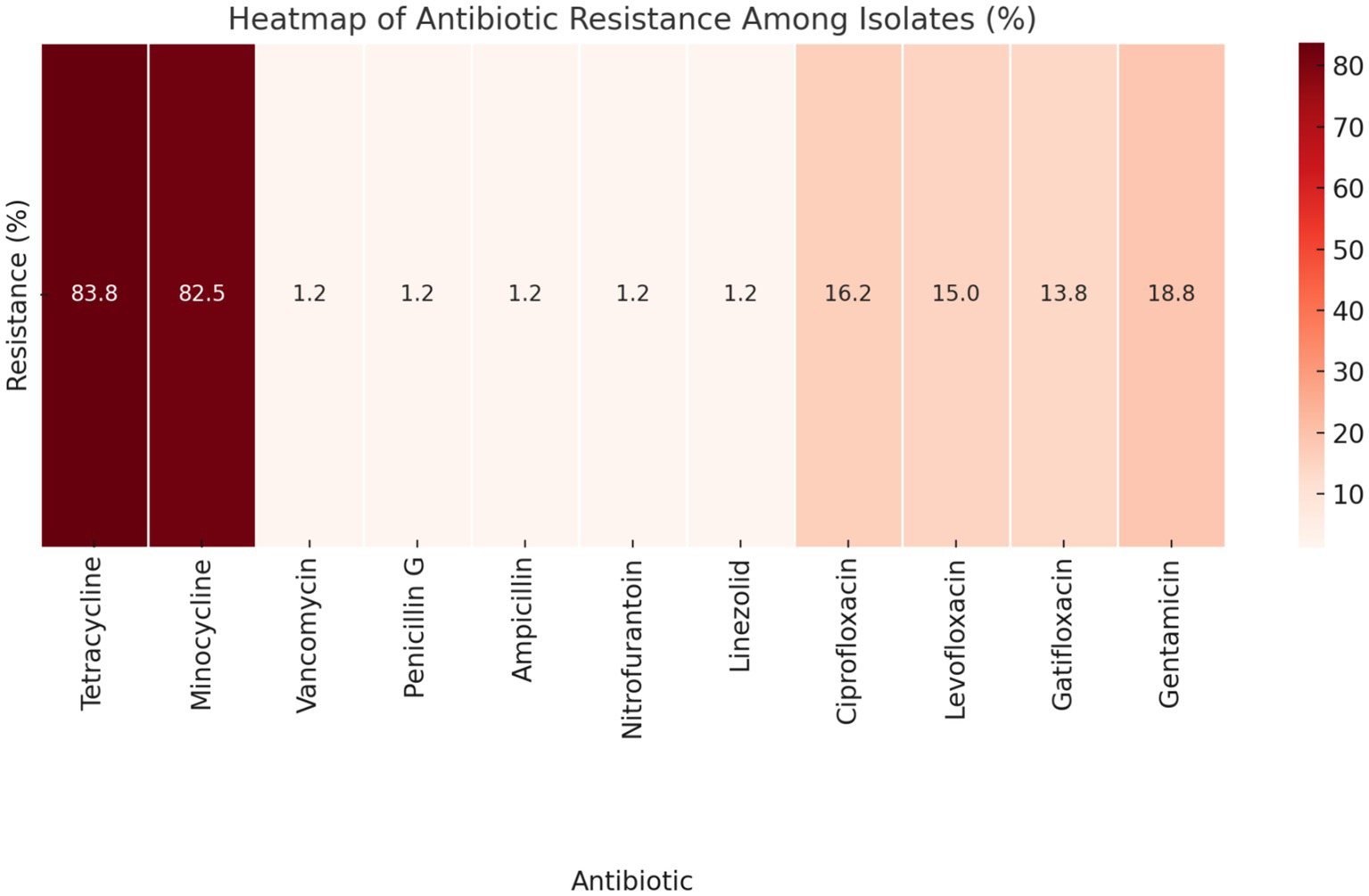
Figure 1. Heatmap displaying the antibiotic resistance patterns among the isolates, according to CLSI interpretation criteria. The darker shades of red indicate higher percentages of resistance, providing a visual representation of how different antibiotics compare in terms of resistance prevalence.
3.3 Presence of virulence geneThe presence of the efaA and ace genes was detected in 93.8% (150/160) of the isolates. For other virulence genes, the distribution was as follows: 72.5% (116/160) of isolates were positive for the esp gene, 61.2% (98/160) for the asa1 gene, 52.5% (84/160) for the cylA gene, and 88.8% (142/160) for the gelE gene.
3.4 Biofilm characteristics: phenotypesBiofilm formation was observed in 25% (40/160) of the isolates. Among these, 15% (24/160) exhibited weak biofilm formation, 8.8% (14/160) displayed moderate biofilm formation, and 1.2% (2/160) demonstrated strong biofilm formation.
3.5 Correlation between biofilm formation and antibiotic resistanceAlthough statistical analysis did not reveal a significant correlation between biofilm formation and antibiotic resistance, some noteworthy patterns emerged (Table 2). Among the isolates tested, a subset of vancomycin-resistant (n = 2), penicillin G-resistant (n = 2), ampicillin-resistant (n = 2), and nitrofurantoin-resistant (n = 2) isolates exhibited strong biofilm formation. Additionally, tetracycline-resistant isolates demonstrated a range of biofilm production: 16 isolates were classified as weak biofilm producers, 14 as moderate, and 2 as strong biofilm producers. Similarly, minocycline-resistant isolates were categorized as 14 weak, 14 moderate, and 2 strong biofilm producers. For ciprofloxacin, levofloxacin, gatifloxacin, and gentamicin resistance, patterns of biofilm formation included 8 weak, 2 moderate, and 2 strong biofilm producers (Figure 2). Due to the limited number of linezolid-resistant isolates, the relationship between linezolid resistance and biofilm formation remains inconclusive. These observations indicate potential associations that warrant further investigation to establish a definitive link.
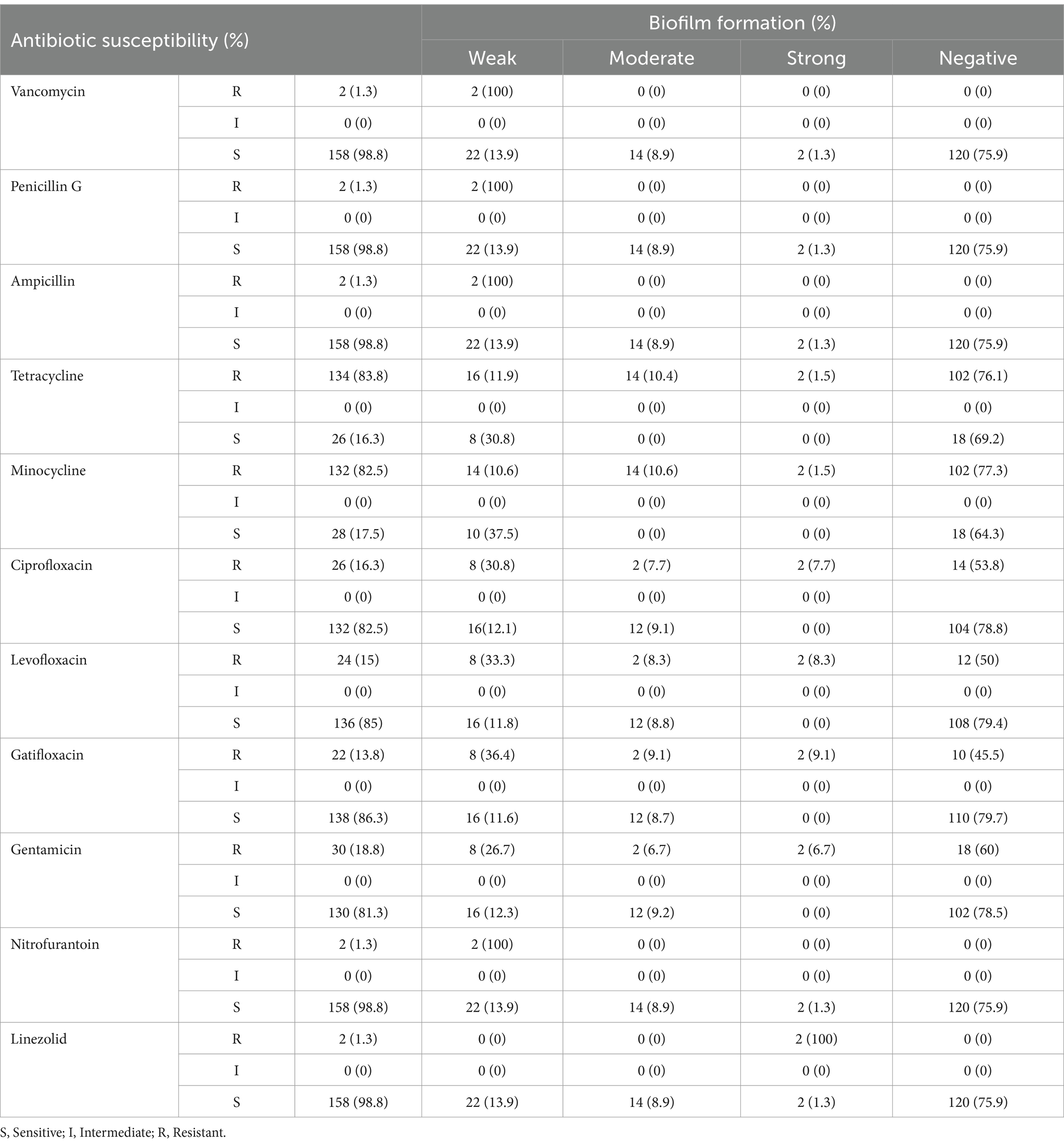
Table 2. The correlation between biofilm formation and distribution of antibiotic resistance in the isolates.
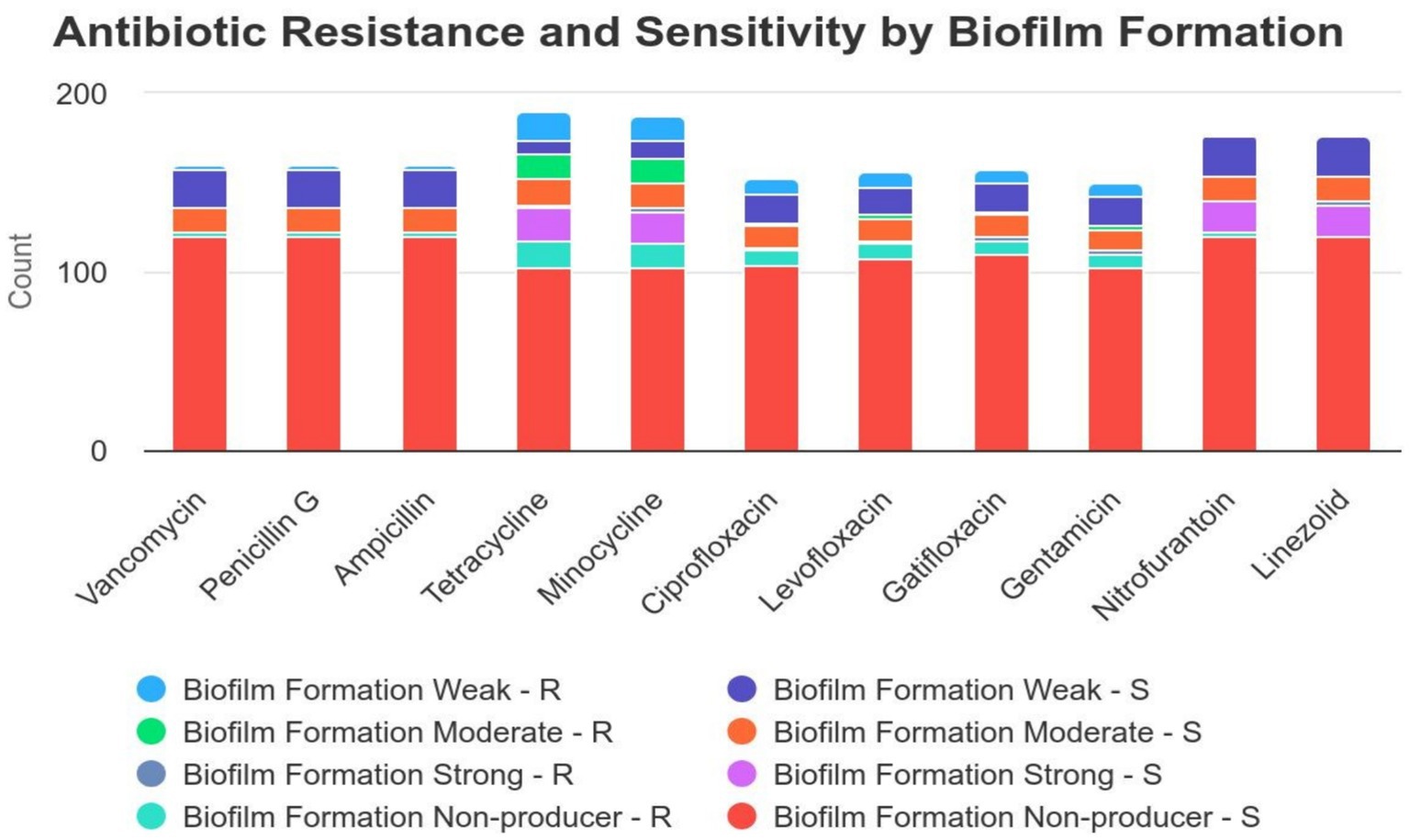
Figure 2. Correlation between biofilm formation and antibiotic resistance distribution in isolates. This figure illustrates the antibiotic resistance and sensitivity patterns among bacterial isolates with different levels of biofilm formation (weak, moderate, strong, and non-producers). The data suggest that isolates with strong biofilm formation exhibit higher resistance to several antibiotics, particularly tetracycline, minocycline, and gentamicin, compared to non-biofilm producers and weak biofilm formers. In contrast, antibiotics such as vancomycin, penicillin G, and nitrofurantoin show low resistance rates across all biofilm formation categories. These findings emphasize the significant role of biofilm formation in increasing bacterial resistance to antibiotics, which is a critical factor to consider when selecting appropriate treatments for infections involving biofilm-forming bacteria.
3.6 The correlation between enterococcal virulence gene distribution and biofilm formationThis study examined the potential association between biofilm formation and the presence of enterococcal virulence genes, as summarized in Table 3. Although statistical analysis did not reveal a significant correlation between biofilm formation and the presence of these virulence genes, several patterns were observed. Specifically, isolates harboring the esp gene (16 isolates), cyl gene (8 isolates), asa1 gene (16 isolates), efbA gene (24 isolates), ace gene (24 isolates), and gelE gene (22 isolates) were predominantly weak biofilm producers. Conversely, isolates with the esp gene (12 isolates), cyl gene (10 isolates), asa1 gene (8 isolates), efbA gene (12 isolates), ace gene (12 isolates), and gelE gene (10 isolates) displayed moderate biofilm formation. Notably, two isolates that exhibited the full complement of tested virulence genes (esp., cyl, asa1, efbA, ace, and gelE) were identified as strong biofilm producers (Figure 3). These observations suggest potential associations that warrant further investigation to elucidate the underlying mechanisms.

Table 3. The correlation between the formation of biofilm and the distribution of virulence genes of E. faecalis in the isolates.
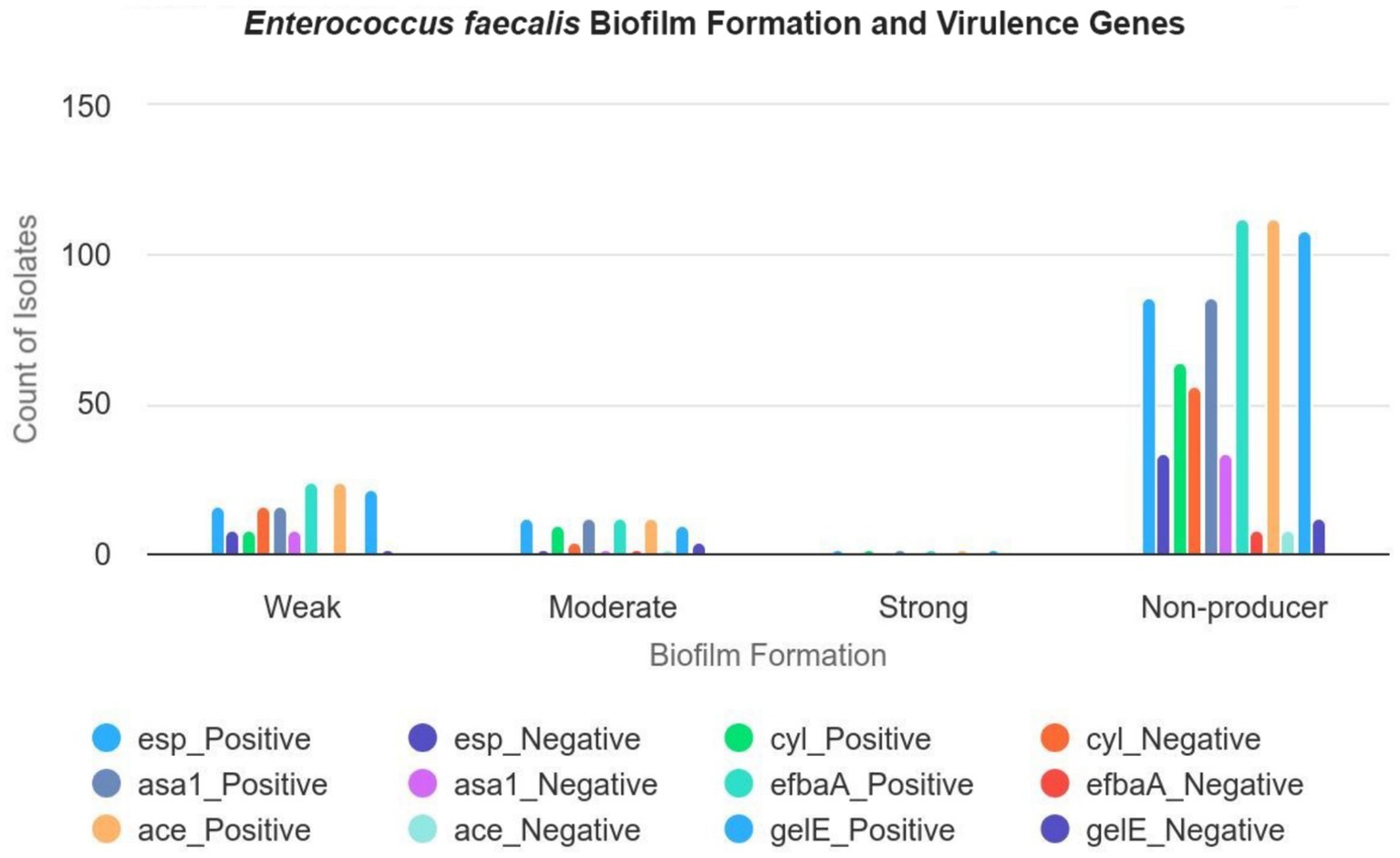
Figure 3. The correlation between the formation of biofilm and the distribution of virulence genes of E. faecalis in the isolates. This figure illustrates the relationship between biofilm formation levels (weak, moderate, strong, and negative) and the presence of various virulence genes (esp., cyl, asa1, efbaA, ace, and gelE) in E. faecalis isolates, with a total of 160 isolates. Weak biofilm formers: Out of 24 isolates, 16 carry the esp gene, 16 carry the asa1 gene, and 16 carry the ace gene. All 24 isolates exhibit the efbaA and gelE genes. Moderate biofilm formers: Out of 14 isolates, 12 carry the esp gene, 10 carry the cyl gene, and 8 carry the asa1 gene. All 14 isolates carry the efbaA, ace, and gelE genes. Strong biofilm formers: Out of 2 isolates, both carry each of the six virulence genes (esp., cyl, asa1, efbaA, ace, and gelE). Negative biofilm formers: Out of 120 isolates, 86 carry the esp gene, 64 carry the cyl gene, and 72 carry the asa1 gene. 112 carry the efbaA and ace genes.
3.7 MLST analysisThe MLST analysis revealed that the two linezolid-resistant Enterococcus faecalis isolates, both identified as strong biofilm producers, were of the same sequence type (ST). Specifically, these isolates were classified as ST150, with the following allelic profile: 3, 6, 23, 12, 1, 10, 7.
4 DiscussionE. faecalis is recognized as a significant Gram-positive pathogen in UTIs, exhibiting notable resistance to a range of commonly used antibiotics such as macrolides and cephalosporins. This resistance arises from both intrinsic factors and acquired mechanisms (Gilmore et al., 2020). Our study highlights a substantial prevalence of antimicrobial resistance among clinical isolates of E. faecalis from UTIs, with particularly pronounced resistance observed against minocycline and tetracyclines. Notably, all E. faecalis isolates, with the exception of two resistant to linezolid, maintained susceptibility to vancomycin, ampicillin, penicillin G, and nitrofurantoin.
These findings are consistent with the reports by Ma et al. (2021) and Chen et al. (2017), who also noted an increase in resistance to minocycline and tetracyclines while finding that all isolates were susceptible to vancomycin and ampicillin. This observation suggests minimal cross-resistance between linezolid and other antibiotics in the E. faecalis isolates studied.
A global perspective on linezolid resistance, as indicated by Dadash et al., reveals that while linezolid resistance is generally low, it exhibits significant regional variability, with higher prevalence observed in Asia compared to other regions (Dadashi et al., 2021). These data corroborate our findings and underscore the importance of localized surveillance in effectively understanding and addressing resistance patterns. The regional variability emphasizes the need for tailored approaches in managing antimicrobial resistance and highlights the value of region-specific data in formulating effective treatment strategies.
Recent research has highlighted the frequent use of antibiotics such as aminoglycosides and nitrofurantoin in the treatment of UTIs caused by vancomycin-resistant E. faecalis (Zhanel et al., 2001; Meena et al., 2017; Levitus et al., 2023). This prevalent exposure may exert selective pressure that contributes to the emergence and persistence of resistant E. faecalis strains. These observations underscore the critical need for stringent antibiotic stewardship to curb the development of resistance. In our study, we observed a relatively lower rate of resistance to vancomycin and nitrofurantoin compared to findings reported by Tripathi et al. (2016) and Meena et al. (2017). These studies document a troubling increase in resistance to these essential antibiotics, which are crucial for managing nosocomial enterococcal infections. The observed discrepancy in resistance patterns highlights the importance of continuous surveillance and research to adapt treatment strategies effectively and maintain the efficacy of these key antimicrobial agents. The divergence between our findings and those in the literature emphasizes the necessity for vigilant monitoring of resistance trends to vancomycin and nitrofurantoin. Such efforts are vital for ensuring the continued availability of effective therapeutic options for managing severe and complex UTIs (Rahbar et al., 2007).
The widespread use of antimicrobial agents has led to a notable rise in multidrug-resistant (MDR) Gram-positive bacteria, posing significant challenges in clinical settings (Patel et al., 2013). Linezolid, a last-resort antimicrobial for Gram-positive infections, has become a cornerstone in treating such resistant strains (Koulenti et al., 2020). However, the increasing use of linezolid has spurred the emergence of linezolid-resistant strains. Our study identified a linezolid resistance rate of 1.2% (2/160) among E. faecalis isolates, which is lower compared to the 3.5 and 3.4% reported by Chen et al. (2018) and Wang et al. (2021) respectively. Moreover, the finding that 1.8% of vancomycin-resistant E. faecalis isolates were also resistant to linezolid underscores a critical limitation in treatment options (Cho et al., 2018). Alarmingly, all linezolid-resistant isolates in our study were also vancomycin-resistant, indicating a potential crisis in managing these infections.
The detection of ST150 in our inpatients suggests its potential adaptation to the hospital environment and acquisition of multidrug resistance. The presence of ST150 in a clinical setting raises concerns about its potential as a problematic strain, especially given its broad-spectrum antibiotic resistance. Recent findings indicate that strains from high-risk clonal complexes (CCs), associated with human infections, have also been found in animals (Freitas et al., 2011). This underlines the need for targeted research into ST150’s genetic mechanisms and its impact on clinical outcomes to develop effective interventions and mitigate its dissemination in healthcare settings (Ma et al., 2021).
Biofilm formation by E. faecalis in UTIs is a significant concern, especially in the context of catheter use. Our study observed that 15% of isolates showed weak, 8.8% moderate, and 1.2% strong biofilm formation. Notably, the two isolates with strong biofilm-forming abilities were ST150 and resistant to all tested antibiotics. This suggests that strong biofilm formation, coupled with extensive antibiotic resistance, could exacerbate infection management challenges.
The lower prevalence of E. faecalis biofilm formation (25%) in our study compared to previous reports (60–90%) in Europe (Sandoe et al., 2003; Arciola et al., 2008; Duprè et al., 2003) could be due to variations in strain sequence types or methodological differences in biofilm assessment. Factors such as strain variability, operational errors in the microtiter plate assay, and lack of standardized biofilm positivity criteria might contribute to these discrepancies.
The pathogenesis of E. faecalis in UTIs involves factors beyond antibiotic resistance, such as colonization, tissue destruction, and evasion of host immune responses. In this study, 93.8% of isolates possessed the efaA and ace genes. Other virulence genes were present at the following rates: esp (72.5%), asa1 (61.2%), cylA (52.5%), and gelE (88.8%). These findings align with previously reported data: 98, 100, and 92.6% for the ace gene in Poland (Łysakowska et al., 2012), and 90, 89.9, and 92.6% for the gelE gene in Italy (Creti et al., 2004) and Iran (Kafil et al., 2013).
The high prevalence of the efbA gene among our isolates underscores its importance in UTI virulence. EfbA facilitates adherence to extracellular matrix (ECM) proteins, crucial for virulence in ascending UTI models. The Ace protein also binds to ECM proteins, aiding in early-stage colonization. Gelatinase (gelE) plays a role in bacterial dissemination by degrading fibrin (Karimi et al., 2018). The esp gene was found in 72.5% of strains, comparable to rates in Iran (77.9%) (Gulhan et al., 2015), Italy (66.7%) (Creti et al., 2004), India (81%) (Singhal et al., 2014), and Japan (72.2%) (Seno et al., 2005), indicating its role as an adhesin. The asa1 gene was present in 61.2% of isolates, similar to rates reported in Iran (69.6%) (Arbabi et al., 2016) and Italy (51%) (Creti et al., 2004). The cylA gene was identified in 52.5% of strains, consistent with findings in Iran (Nasaj et al., 2016), Japan (Seno et al., 2005), and India (Gupta et al., 2014). The predominance of virulence determinants such as efaA, ace, and gelE in our isolates underscores their significant role in E. faecalis pathogenicity. The high prevalence of these factors in our study, compared to others, highlights the need for ongoing surveillance and research. The presence of multiple virulence factors in our isolates suggests a complex interplay between resistance and pathogenicity that warrants further investigation.
In summary, our findings underscore the need for continuous monitoring of antimicrobial resistance and virulence factors in E. faecalis. The identification of ST150 and its associated resistance profile, coupled with biofilm-forming capabilities, points to a critical area for future research and intervention. Addressing these challenges will be essential for improving clinical outcomes and managing resistant infections effectively.
5 ConclusionThis study highlights the emergence of the ST150 clonal lineage of Enterococcus faecalis in Tehran, Iran, with a focus on its role in urinary tract infections (UTIs). The data indicate a significant presence of E. faecalis in UTIs, with high resistance rates to tetracycline and minocycline, while maintaining high susceptibility to vancomycin, penicillin G, ampicillin, and nitrofurantoin. Notably, a small percentage of isolates demonstrated resistance to linezolid, with these resistant strains belonging to the previously unreported ST150 lineage. The presence of various virulence factors and the ability to form biofilms among these isolates underline their pathogenic potential. Although no definitive correlation between biofilm formation and antibiotic resistance was found, patterns suggest that biofilm production might be associated with resistance. The study underscores the importance of continuous surveillance and molecular characterization of E. faecalis to better understand and address emerging resistance patterns and enhance infection control strategies.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statementThe studies involving humans were performed in accordance with the ethical standards of Azad University, Tehran, Iran and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The participants provided their written informed consent to participate in this study.
Author contributionsMS: Investigation, Methodology, Writing – original draft. MK: Writing – review & editing. FG: Project administration, Supervision, Writing – review & editing. MA: Software, Writing – original draft. SA: Methodology, Writing – original draft. NB: Methodology, Project administration, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsImages and charts in this article were generated using the Highcharts GPT v11.4.8 Generative AI platform, available at https://www.highcharts.com/chat/gpt/. The tool was used to design custom visualizations and charts based on the input data. The platform’s generative AI capabilities were used to produce interactive and static charts, ensuring that the results were consistent with the study’s data and requirements.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAbdullah, H. H., Saleh, S. S., Hassan, Z. I., and Uso, R. S. (2023). Antibiotic resistance patterns in individuals with urinary tract infections: bacterial profile. J. Adv. Zool. 44, 1406–1420. doi: 10.53555/jaz.v44iS2.976
Crossref Full Text | Google Scholar
Arbabi, L., Boustanshenas, M., Rahbar, M., Owlia, P., Adabi, M., Koohi, S. R., et al. (2016). Antibiotic susceptibility pattern and virulence genes in Enterococcus spp. isolated from clinical samples of Milad hospital of Tehran, Iran. Archives of. Clin. Infect. Dis. 11:260. doi: 10.5812/archcid.36260
Crossref Full Text | Google Scholar
Arciola, C. R., Baldassarri, L., Campoccia, D., Creti, R., Pirini, V., Huebner, J., et al. (2008). Strong biofilm production, antibiotic multi-resistance and high gelE expression in epidemic clones of Enterococcus faecalis from orthopaedic implant infections. Biomaterials 29, 580–586. doi: 10.1016/j.biomaterials.2007.10.008
Crossref Full Text | Google Scholar
Armin, S., Fallah, F., Karimi, A., Rashidan, M., Shirdust, M., and Azimi, L. (2017). Genotyping, antimicrobial resistance and virulence factor gene profiles of vancomycin resistance Enterococcus faecalis isolated from blood culture. Microb. Pathog. 109, 300–304. doi: 10.1016/j.micpath.2017.05.039
Crossref Full Text | Google Scholar
Aung, M. S., Urushibara, N., Kawaguchiya, M., Ohashi, N., Hirose, M., Kudo, K., et al. (2023). Antimicrobial resistance, virulence factors, and genotypes of Enterococcus faecalis and Enterococcus faecium clinical isolates in northern Japan: identification of optrA in ST480 E. faecalis. Antibiotics 12:108. doi: 10.3390/antibiotics12010108
Crossref Full Text | Google Scholar
Bai, B., Hu, K., Li, H., Yao, W., Li, D., Chen, Z., et al. (2018). Effect of tedizolid on clinical Enterococcus isolates: in vitro activity, distribution of virulence factor, resistance genes and multilocus sequence typing. FEMS Microbiol. Lett. 365:284. doi: 10.1093/femsle/fnx284
Crossref Full Text | Google Scholar
Bostanghadiri, N., Ghalavand, Z., Fallah, F., Yadegar, A., Ardebili, A., Tarashi, S., et al. (2019). Characterization of phenotypic and genotypic diversity of Stenotrophomonas maltophilia strains isolated from selected hospitals in Iran 10, 1191. doi: 10.3389/fmicb.2019.01191
Crossref Full Text | Google Scholar
Chen, C.-H., Lin, L.-C., Chang, Y.-J., and Chang, C.-Y. (2017). Clinical and microbiological characteristics of vancomycin-resistant Enterococcus faecium bloodstream infection in Central Taiwan. Medicine 96:e9000. doi: 10.1097/MD.0000000000009000
Crossref Full Text | Google Scholar
Chen, M., Pan, H., Lou, Y., Wu, Z., Zhang, J., Huang, Y., et al. (2018). Epidemiological characteristics and genetic structure of linezolid-resistant Enterococcus faecalis. Infect. Drug Resist. 11, 2397–2409. doi: 10.2147/IDR.S181339
Crossref Full Text | Google Scholar
Cho, S. Y., Kim, H. M., Chung, D. R., Kim, S. H., Huh, H. J., Kang, C.-I., et al. (2018). Resistance mechanisms and clinical characteristics of linezolid-resistant Enterococcus faecium isolates: a single-Centre study in South Korea. J. Glob. Antimicrob. Resist. 12, 44–47. doi: 10.1016/j.jgar.2017.09.009
Crossref Full Text | Google Scholar
Codelia-Anjum, A., Lerner, L. B., Elterman, D., Zorn, K. C., Bhojani, N., and Chughtai, B. J. A. (2023). Enterococcal urinary tract infections: a review of the pathogenicity. Epidemiol. Treat. 12:778. doi: 10.3390/antibiotics12040778
Crossref Full Text | Google Scholar
Comerlato, C. B., Resende, M. C. C., Caierão, J., and d'Azevedo, P. A. (2013). Presence of virulence factors in Enterococcus faecalis and Enterococcus faecium susceptible and resistant to vancomycin. Mem. Inst. Oswaldo Cruz 108, 590–595. doi: 10.1590/S0074-02762013000500009
Crossref Full Text | Google Scholar
Creti, R., Imperi, M., Bertuccini, L., Fabretti, F., Orefici, G., Di Rosa, R., et al. (2004). Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J. Med. Microbiol. 53, 13–20. doi: 10.1099/jmm.0.05353-0
PubMed Abstract | Crossref Full Text | Google Scholar
Dadashi, M., Sharifian, P., Bostanshirin, N., Hajikhani, B., Bostanghadiri, N., Khosravi-Dehaghi, N., et al. (2021). The global prevalence of daptomycin, tigecycline, and linezolid-resistant Enterococcus faecalis and Enterococcus faecium strains from human clinical samples: a systematic review and meta-analysis. Front. Med. 8:720647. doi: 10.3389/fmed.2021.720647
Crossref Full Text | Google Scholar
Duprè, I., Zanetti, S., Schito, A. M., Fadda, G., and Sechi, L. A. (2003). Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy). J. Med. Microbiol. 52, 491–498. doi: 10.1099/jmm.0.05038-0
PubMed Abstract | Crossref Full Text | Google Scholar
Freitas, A. R., Coque, T. M., Novais, C., Hammerum, A. M., Lester, C. H., Zervos, M. J., et al. (2011). Human and swine hosts share vancomycin-resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 clonal clusters harboring Tn1546 on indistinguishable plasmids. J. Clin. Microbiol. 49, 925–931. doi: 10.1128/JCM.01750-10
Crossref Full Text | Google Scholar
Gilmore, M. S., Salamzade, R., Selleck, E., Bryan, N., Mello, S. S., Manson, A. L., et al. (2020). Genes contributing to the unique biology and intrinsic antibiotic resistance of Enterococcus faecalis. mBio 11:6. doi: 10.1128/mBio.02962-20
Crossref Full Text | Google Scholar
Govindarajan, D. K., and Kandaswamy, K. (2022). Virulence factors of uropathogens and their role in host pathogen interactions. Cell Surface 8:100075. doi: 10.1016/j.tcsw.2022.100075
Crossref Full Text | Google Scholar
Govindarajan, D. K., Meghanathan, Y., Sivaramakrishnan, M., Kothandan, R., Muthusamy, A., Seviour, T. W., et al. (2022). Enterococcus faecalis thrives in dual-species biofilm models under iron-rich conditions. Arch. Microbiol. 204:710. doi: 10.1007/s00203-022-03309-7
Crossref Full Text | Google Scholar
Gulhan, T., Boynukara, B., Çiftçi, A., Sogut, M., and Findik, A. (2015). Characterization of Enterococcus faecalis isolates originating from different sources for their virulence factors and genes, antibiotic resistance patterns, genotypes and biofilm production. Iran. J. Vet. Res. 16, 261–266
PubMed Abstract | Google Scholar
Gupta, S., Kapur, S., and Padmavathi, D. (2014). Comparative prevalence of antimicrobial resistance in community-acquired urinary tract infection cases from representative states of northern and southern India. J. Clin. Diagn. Res. 8:DC09. doi: 10.7860/JCDR/2014/9349.4889
Crossref Full Text | Google Scholar
Jahansepas, A., Ahangarzadeh Rezaee, M., Hasani, A., Sharifi, Y., Rahnamaye Farzami, M., Dolatyar, A., et al. (2018). Molecular epidemiology of vancomycin–resistant Enterococcus faecalis and Enterococcus faecium isolated from clinical specimens in the northwest of Iran. Microb. Drug Resist. 24, 1165–1173. doi: 10.1089/mdr.2017.0380
PubMed Abstract | Crossref Full Text | Google Scholar
Kafil, H. S., Mobarez, A. M., and Moghadam, M. F. (2013). Adhesion and virulence factor properties of enterococci isolated from clinical samples in Iran. Indian J. Pathol. Microbiol. 56, 238–242. doi: 10.4103/0377-4929.120375
Comments (0)