The neuronal phenomenon spreading depolarization (SD) is characterized by a massive depolarization of neurons and glial cells (Müller and Somjen, 2000), a near-complete breakdown of intra/extracellular ion homeostasis and a potentially reversible impairment of neuronal network function (Leão, 1944; Marshall, 1959; Bures et al., 1974). In vivo, spreading depolarization triggers a rapidly evolving reduction in the amplitudes of spontaneous activity termed spreading depression. The induction of SD follows an all-or-none principle (Somjen, 2001), and once ignited, it may spread out slowly across the gray matter in a wave-like fashion. In principle, SD may arise in most regions of the central nervous system, yet with clearly differing induction thresholds. Neocortex and hippocampus are more prone to SD than cerebellum, spinal cord, olfactory bulb or brainstem (Fifkova et al., 1961; Amemori et al., 1987; Richter et al., 2003; Funke et al., 2009). SD is triggered by various stimuli, such as increased extracellular K+, electrical stimulation, or hyperthermia. A generally recognized crucial component in the process of SD induction is the challenge of the Na+/K+ ATPase. This becomes particularly clear with trigger factors such as disturbed energy-supply or energy-utilization, including O2 withdrawal, combined O2 glucose withdrawal, pharmacological inhibition of Na+/K+ ATPase, or mitochondrial poisoning/uncoupling (Andrew et al., 2022b).
The clinical interest was sparked, when it became clear that SD is not limited to ex vivo experimental preparations, but may affect also the (intact) human brain. SD is considered in the context of various neurological disorders and conditions, such as migraine, commotio cerebri, postictal depression, cerebral hypoxia/ischemia, and cerebral hemorrhage (van Harreveld and Stamm, 1954; Lauritzen, 1994; Dreier et al., 2006; Dohmen et al., 2008). Furthermore, it is involved in the development of cortical lesions associated with neurological pathologies (Hartings et al., 2017). For example, a recent case series using continuous invasive neuromonitoring combined with longitudinal neuroimaging has demonstrated the prominent role of SD in humans not only temporally after the manifestation of ischemic brain infarcts, but also before and during their development (Lückl et al., 2018). Unclear remains, however, to what extent reactive oxygen species (ROS) are generated during SD and which particular cellular mechanisms participate.
At the cellular level SD evokes a marked flux of K+ into the extracellular space, followed by a massive influx of Na+, Ca2+ and Cl−, causing near complete depolarization and swelling of neurons and glial cells (Kraig and Nicholson, 1978; Hansen and Olsen, 1980; Hansen and Zeuthen, 1981). In this condition, which has been termed cytotoxic edema, the electrical resistance of the tissue increases and profound ultrastructural changes occur especially in the dendrites (van Harreveld and Ochs, 1957; Dreier et al., 2018; Kirov et al., 2020). Multiple brief SDs are tolerated by brain tissue without challenging neuronal viability (Nedergaard and Hansen, 1988), but prolonged SD eventually causes neuronal death (Kawasaki et al., 1988). In this process, cellular Ca2+-load and metabolic stress seem crucial (Jing et al., 1991; Hartings et al., 2017; Andrew et al., 2022a). However, redox alterations and ROS formation may also need to be considered (Andrew et al., 2022a).
By distorting ionic homeostasis, SD evokes a serious metabolic burden, as indicated by markedly elevated glucose utilization (Shinohara et al., 1979), mitochondrial depolarization (Bahar et al., 2000) and a depletion of ATP on the tissue (Mies and Paschen, 1984) and cellular/neuronal level (unrefereed preprint: Schoknecht, K., Baeza-Lehnert, F., Hirrlinger, J., Dreier, J. P., Eilers J. (2024). Spreading depolarizations exhaust neuronal ATP in a model of cerebral ischemia. bioRxiv, 2024: 605834. doi: https://doi.org/10.1101/2024.07.30.605834). This metabolic burden may then contribute to intensified ROS formation. Indeed, antioxidants (AO) may protect against SD induction, suggesting that ROS may modulate SD induction and/or propagation (Grinberg et al., 2012; Mendes-da-Silva et al., 2014). Direct proof of ROS formation and oxidative stress during SD was obtained in mouse hippocampal slice-cultures by CellROX imaging upon electrically-evoked SD (Grinberg et al., 2012) and in rat neocortex by measuring lipid peroxidation upon K+-induced SD (Shatillo et al., 2013), recording MitoSOX fluorescence during brain- trauma-induced SDs (Aboghazleh et al., 2021), or quantifying superoxide dismutase activity together with HPLC analysis of H2O2 contents in microdialysates upon K+-induced SD (Viggiano et al., 2011). For technical reasons, these analyses assessed, however, only a single time point after the tissue had undergone SD. Therefore, the detailed spatiotemporal dynamics of ROS formation during SD remained unclear. A first time-resolved assessment of ROS formation associated with cortical spreading ischemia and cortical spreading depression was based on lucigenin chemiluminescence in the cortex of anesthetized rats, confirming superoxide formation as a consequence of spreading ischemia but not K+-induced spreading depression (Dreier et al., 1998).
An intact redox balance is essential for the maintenance of cellular homeostasis. This fragile equilibrium may be distorted, however, by excessive ROS generation, thereby modulating cellular responsiveness, challenging proper cell function or even cellular viability. Based on reactivity profiling, 890 human proteins may be functionally controlled by thiol-based redox modulation (Weerapana et al., 2010). As we demonstrated earlier, also the susceptibility to SD is modulated by redox alterations, with sulfhydryl-reducing conditions favoring and sulfhydryl-oxidizing conditions postponing the onset of hypoxia-induced SD (HSD) (Hepp et al., 2005; Hepp and Müller, 2008). It therefore seems worthwhile taking a closer look at ROS generation and neuronal redox alterations during SD.
The reliable monitoring of subcellular redox conditions in living tissue demands state-of-the-art optical redox sensors, such as the genetically-encoded reduction/oxidation sensitive green fluorescent proteins (roGFPs), which respond reversibly to oxidation as well as reduction and can be expressed in different cell compartments (Hanson et al., 2004). Furthermore, their excitation-ratiometric properties enable quantitative analyses and a reliable comparison of different preparations and conditions (Meyer and Dick, 2010). Mechanistically, engineered reactive thiols respond to ambient redox conditions. Closing (oxidizing) or opening (reducing) of disulfide bridges induces conformational changes, thereby affecting light absorbance and hence fluorescence emission of roGFP (Hanson et al., 2004). Thereby, dynamic and real-time measurements of cellular redox balance become possible. In detailed tests we confirmed the reliability of roGFP in neuronal preparations (Funke et al., 2011; Can et al., 2017) and finally generated transgenic redox indicator mice stably expressing roGFP1 either within cytosol (roGFPc mice, B6J-Tg(Thy1.2-roGFP1c)2Mmllr) or mitochondrial matrix (roGFPm mice, B6J-Tg(Thy1.2-roGFP1m)1Mmllr) of excitatory projection neurons (Wagener et al., 2016). Using these mice, we here deciphered for a first time SD-related neuronal redox alterations in real time and in a quantitative manner. This clearly proved that different modes of SD are accompanied by a massive neuronal oxidation, which shows a clear Ca2+ dependence and involves the generation of ROS by mitochondria as well as non-mitochondrial sources.
2 Materials and methods 2.1 PreparationAcute cortico-hippocampal tissue slices were obtained from adult male roGFP mice (Wagener et al., 2016); their average age was 5.8 ± 2.9 months (n = 65). Mice were kept and bred at the central animal facility of the University Medical Center Göttingen. For AO treatment, they received an AO-enriched diet upon weaning. The regular diet (V1124-0, SSNIFF Spezialdiäten, Soest, Germany) was supplemented with 250 mg/kg diet α-lipoic acid, 2.5 g/kg diet N-acetylcysteine, and an additional 125 mg/kg diet vitamin E (α-tocopherol).
Mice were decapitated under deep anesthesia (ether or isoflurane), the brain was isolated and submerged in ice-cold artificial cerebrospinal fluid (ACSF) for 2–3 min. Next, 350 μm-thick coronal tissue slices were cut (752 M Vibroslice, Campden Instruments) and divided in the sagittal midline. These hemi-slices were submerged in a storage chamber with oxygenated regular ACSF (20–22°C) for ≥90 min before the respective recordings were started. Combined electrophysiological and fluorescence recordings were conducted in a submersion-style chamber, which was custom-made by our mechanical workshop to meet the specific technical requirements of our experiments (Figure 1A). The chamber was heated to 35.5–36.0°C and constantly supplied with fresh oxygenated ACSF.
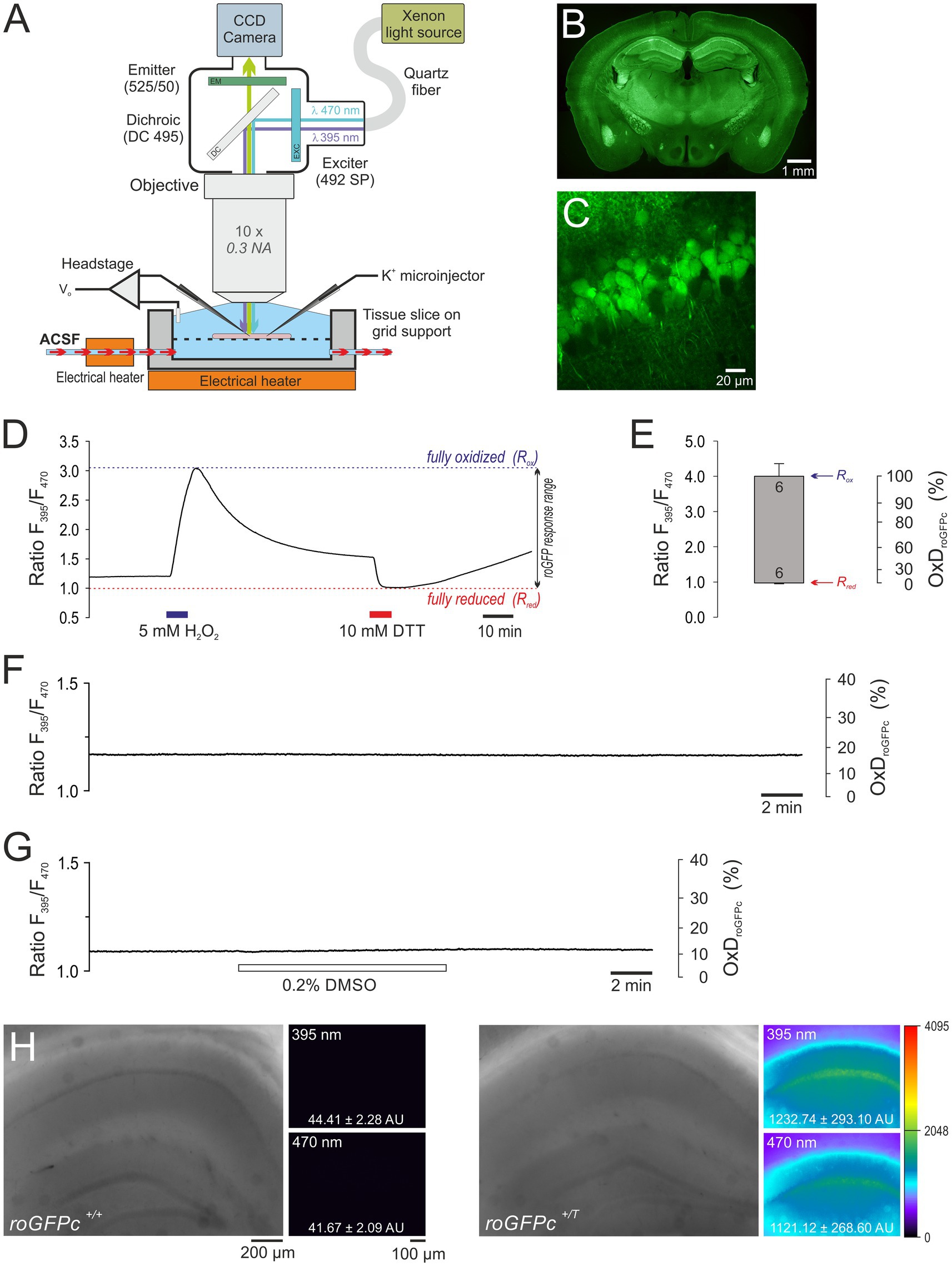
Figure 1. Combined electrophysiological and optical redox imaging experiments in acute slices from roGFPc redox indicator mice. (A) Sensitive detection of roGFP fluorescence demands an immersion objective. Thus we were facing the challenge of eliciting SD in submerged brain tissue slices. To ensure tissue viability at the required experimental temperature of 35.5–36.0°C, high flow rates of ACSF (8 mL/min), a slice thickness of 350 μm, and unrestricted access of fresh solution to the upper and lower slice surface turned out to be crucial. SD was triggered by either O2 withdrawal, mitochondrial uncoupling or K+ microinjection into the slice. (B) PFA-fixed transverse slice (30 μm thickness) of a female roGFPc mouse (6 month old) showing the widespread expression of the redox indicator. Note the particularly high expression in hippocampus and the CA1 subfield. The displayed overview is a stitched composition of 12 smaller images; roGFPc fluorescence is displayed in pseudo-colors. (C) Close-up view of the CA1 pyramidal cell layer confirming the expression of roGFPc in virtually every pyramidal neuron. Image was taken from an acute brain tissue slice (400 μm thickness, 3 weeks old female roGFPc mouse) with a 2-photon imaging system (TrimScope 2, LaVision BioTec) at 740 nm excitation and 195 nm/pixel resolution. (D) Response calibration of roGFPc recorded in stratum pyramidale of the hippocampal CA1 subfield. It represents the maximum oxidation (Rox) and reduction (Rred) of roGFPc induced by exposure to H2O2 and DTT, respectively. During calibration, sufficiently long wash-out and recovery times are essential to prevent direct interactions of the two redox-active compounds. (E) Determined roGFPc response range. The upper margin of the box represents the averaged Rox, the lower margin the averaged Rred. Error bars represent the respective standard deviations; the number of slices measured is indicated. Based on the determined calibration parameters, the average degree of roGFPc oxidation (OxDroGFPc) is calculated for quantitative redox analysis. (F) Control recording confirming that repeated illumination per se does not evoke any obvious changes in roGFPc ratio. In the displayed experiment a 3-fold increased frame rate of 0.33 Hz was applied. (G) The solvent DMSO (0.2%, 15 min) did not markedly affect roGFPc fluorescence. (H) Comparing slices from roGFPc transgenic (roGFPc+/T) and non-transgenic (roGFPc+/+) male mice (5 months old siblings) confirms that a contribution of tissue autofluorescence is of no concern. Hardly any fluorescence could be detected in non-transgenic slices. Overview images were taken with an 5x objective and white light illumination. Fluorescence images were acquired under imaging conditions using the 10x water immersion objective. Excitation wavelengths are indicated, and the averaged pixel intensities of the entire fluorescence image are reported in arbitrary units (AU). Fluorescence intensities are displayed in a 12 bit pseudocolor palette spanning 4,096 intensity levels.
2.2 SolutionsUnless stated differently, chemicals were obtained from Sigma-Aldrich. Regular ACSF contained (in mM): 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 1.2 CaCl2, 1.2 MgSO4, and 10 dextrose. The conditioning ACSF used to facilitate SD induction contained a moderately increased K+ concentration (8 mM) and was slightly hypo-osmolar (only 110 mM NaCl) (Huang et al., 1997; Funke et al., 2009). In nominally-free Ca2+ solutions, CaCl2 was omitted from the ACSF. All solutions were constantly aerated with carbogen (95% O2, 5% CO2) to maintain pH 7.4. FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, Tocris), and DPI (diphenyleneiodonium, Tocris) were dissolved in DMSO (dimethyl sulfoxide) as 10 mM stocks and kept frozen (−20°C). Final DMSO contents during the experiments were ≤ 0.2%. Allopurinol was directly dissolved in conditioning ACSF shorty before use.
2.3 Optical recordingsQuantitative redox imaging was performed by excitation-ratiometric imaging of roGFP fluorescence (Funke et al., 2011; Wagener et al., 2016). The imaging system was composed of an upright microscope (Axiotech, Zeiss), a switchable xenon light source (TILL Photonics Polychrome V, ThermoFisher Scientific) and a sensitive CCD camera (Imago QE, PCO Imaging). Slices were viewed with a 5×, 0.13NA objective (Epiplan, Zeiss) for proper positioning in the chamber and electrode insertion. For fluorescence imaging, a low magnification 10×, 0.3NA water immersion objective was chosen (Achroplan, Zeiss).
Taking advantage of the rapid wavelength-switching capabilities of the computer-controlled imaging system, roGFP was alternately excited at 395 and 475 nm and the respective fluorescence recorded at the tissue level at low magnification (TILLvision 4.5 software; ThermoFisher Scientific). Exposure times were 12 ms for each wavelength, 2×2 pixel binning was applied, and ratiometric image pairs were recorded every 10 s (0.1 Hz frame rate). The fluorescence ratio F395/F470 was calculated in real-time for pre-defined regions of interest (ROIs, ~20×40 μm) near the recording electrode and displayed as line plots. An increased fluorescence ratio F395/F470 indicates oxidation of roGFP, a decreased ratio indicates reduction of roGFP. Post-experimental image analysis was performed with TILLvision 4.5 and Metamorph offline 7.8 (Molecular Devices).
For quantitative recordings we calibrated the ratiometric roGFPc responses in the CA1 pyramidal cell layer (Figures 1D,E). The ratio representing full oxidation (Rox) was determined by exposing the slice to 5 mM H2O2 (7 min), the ratio representing full roGFP reduction (Rred) was determined by treatment with 10 mM DTT (7 min). Monitoring both responses in the same slice, provides also the ratio of fluorescence intensities (F470ox/F470red). Once calibrated, the relative degrees of roGFP oxidation (OxDroGFP) were then calculated for the roGFP1 sensor under our recording conditions (Meyer and Dick, 2010; Wagener et al., 2016):
OxDroGFP=R−RredF470oxF470redRox−R+R−Rred 2.4 Electrophysiological recordingsExtracellular DC potentials were recorded with a custom-built amplifier and glass microelectrodes inserted in CA1 stratum radiatum. Thin-walled borosilicate glass capillaries (GC150TF-10, Harvard Apparatus) were pulled on a P-97 electrode puller (Sutter Instruments), filled with ACSF, and their tips trimmed to a resistance of ~5 MΩ. DC potentials were sampled/digitized at 100 Hz (Axon-Instruments Digitizer 1322A, PClamp 9.0 software, Molecular Devices) and analyzed offline. In parallel, the TTL-pulses triggering image acquisition by the CCD camera were recorded, to synchronize the optical and electrophysiological signatures of SD during data analysis. In some slices, field excitatory postsynaptic potentials (fEPSPs) were evoked orthodromically every 30 s by 1.0–1.5 mA unipolar stimuli of 0.1 ms duration (S88 Grass Stimulator with PSIU6 stimulus isolation units, 50 μm steel wire electrode). In these recordings, a sampling rate of 20 kHz was used. For reliable stimulation of the submerged tissue slices, the steel microwire electrodes – except for their very tips – were insulated with 3 layers of polyurethane paint (urethan 71, CRC Industries). All DC potential amplitudes were measured between the respective pre-treatment baseline and the nadir of SD. As SD onset we defined the sudden negative DC potential deflection (ΔVo); only rapid deflections were considered as full-blown SDs.
Normoxic SD was induced by local injection of 3 M KCl microdroplets (PDES-02DX microinjector, NPI Electronic, Tamm, Germany; 1.5 s pressure pulse of 0.25 bar). HSD was triggered by 95% N2, 5% CO2 aerated conditioning ACSF including also 2 mM sodium sulfite (Na2SO3) to remove any residual O2 and prevent a re-oxygenation within the tubing system and the recording chamber (Flyvbjerg et al., 1993; Funke et al., 2011). Chemically-induced SDs were evoked by the mitochondrial uncoupler FCCP (2 μM) (Gerich et al., 2006).
2.5 StatisticsReported data were collected from 65 mice, typically using 3–4 slices from each brain. To ensure the independence of observations, each experimental paradigm was conducted on at least 3 different mice. For a first screening of data, an outlier test (ROUT, Q = 1%, GraphPad Prism 9.5.1.) was performed and only those slices were included into analysis, which presented reasonable optical and electrophysiological data in the combined recordings.
Results are reported as mean ± standard deviation, n represents the number of slices analyzed. The scatter of the individual data points is displayed in the diagrams. All treatment-related changes are referred to the respective control conditions. Normal distribution was verified by the Kolmogorov–Smirnov test. The statistical significance of optical/electrophysiological data sets was assessed in one-sample Student’s t-tests at a significance level of 5%, by comparing any evoked differences against pre-treatment control conditions (defined as unity). For the comparison of several groups/treatment conditions, one way ANOVA (analysis of variance) followed by Holm-Šídák comparisons were used. In the case of non-normally distributed data sets, a Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by Dunn’s test comparisons were applied. In the diagrams significant changes are indicated by asterisks (* p < 0.05; ** p < 0.01, *** p < 0.001) and the statistical test applied is stated. For data processing and statistical calculations Excel (Office 2016), Sigma Stat 3.5 (Systat Software), and GraphPad Prism 9.5.1 (GraphPad, Inc.) were used.
3 Results 3.1 Optimizing recording conditions and slice chamber designEstablishing quantitative redox imaging during SD, we were facing the two challenges of efficient fluorescence detection and the high temperature required to induce SD. To guarantee highest possible fluorescence detection sensitivity, the slices had to be submerged. Yet, this condition complicates the maintenance of brain tissue viability and the reliable induction of SD. Therefore, the very first task was the design of an experimental chamber for the electro-optical recordings at the required temperature of 35.5–36.0°C (Figure 1A).
Based on earlier experience and advice of colleagues who regularly study SD under submerged conditions (Reinhart and Shuttleworth, 2018), acute brain tissue slices were prepared at a thickness of 350 μm and positioned on a supportive nylon mesh to assure undisturbed contact of both slice surfaces to fresh ACSF. Also high flow rates of 8 mL ACSF/min were applied to guarantee sufficient O2 supply of the submerged slices (Hájos et al., 2009; Reinhart and Shuttleworth, 2018).
3.2 Validation and calibration of roGFP responsesQuantitative redox imaging demands a proper validation and calibration of the redox sensor in the respective tissue. Based on the expression of roGFPc in the cytosol of excitatory neurons, we chose CA1 stratum pyramidale for all experiments, as it showed the highest roGFP expression (Figures 1B,C). Due to the massive cell swelling during SD, redox imaging was not performed at the single-cell level, but at low magnification in defined tissue volumes, i.e., ROIs of about 20×40 μm size, containing several roGFP-expressing neurons.
At first we calibrated the ratiometric response range (Figures 1D,E) and determined the maximum oxidation (Rox) and maximum reduction (Rred) of roGFPc by exposing the slices to 5 mM H2O2 and 10 mM DTT (7 min each). In detail, an Rox of 4.000 ± 0.370, an Rred of 0.972 ± 0.013, and an instrument factor F470ox/F470red of 0.332 ± 0.035 (n = 6 each) were obtained (Figures 1D,E). Under resting control conditions, the roGFPc ratio within CA1 stratum pyramidale averaged 1.174 ± 0.080 (n = 98). Based on the calibrations, this corresponds to an average degree of roGFPc oxidation (OxDroGFPc) of 17.4 ± 5.8% (n = 98).
Next, we validated the stability and reliability of roGFP under the chosen experimental conditions. Brain tissue slices were exposed to the imaging protocol without any other treatments, and in part the imaging frame rate was even increased from 0.1 Hz to 0.33 Hz. As expected, up to 1,000 cycles of ratiometric excitation did not evoke marked OxDroGFPc alterations in the hippocampal CA1 subfield (2.43 ± 2.44 percentage points as compared to control conditions, n = 4, range − 0.32 – 5.04 percentage points). This confirms that the frequent illumination and the associated recording conditions do not interfere with roGFP integrity and/or the redox conditions in the studied tissue volumes (Figure 1F).
Furthermore, we addressed potential side-effects of the drug solvent DMSO. Any marked effects of DMSO (0.2%, 15 min) on the redox balance could not be observed within the CA1 pyramidal cell layer (n = 3, Figure 1G). Compared to baseline control conditions, OxDroGFPc only slightly increased during the 15 min of DMSO administration by 1.16 ± 0.86 percentage points.
Finally, we ruled out potential contributions of tissue autofluorescence, by imaging wildtype slices devoid of roGFPc (n = 3). With identical 395 nm and 470 nm imaging settings applied, hardly any fluorescence was detectable in non-transgenic slices, confirming that roGFP fluorescence is at least 25-fold more intense than any background autofluorescence of the hippocampal tissue (Figure 1H).
3.3 Spreading depolarization is paralleled by an oxidizing redox shiftTo characterize the redox changes associated with SD, we induced SDs by either an increase in the extracellular K+ concentration, O2 withdrawal or mitochondrial uncoupling, thereby covering a spectrum of normoxic, hypoxia-and chemically-induced SDs. For reliable SD induction, conditioning ACSF was used in all recordings (see solutions).
Normoxic, K+-induced SDs were triggered by local K+ micro injection into the slice (stratum radiatum) ~100 μm next to the electrical recording site. After a short delay of a few seconds (range 3–7 s) SD occurred, showing the characteristic negative extracellular DC potential shift (ΔVo), which averaged −4.6 ± 2.4 mV (n = 13; see Table 1). It was accompanied by a more slowly developing increase in roGFPc ratio, i.e., an oxidizing shift in cytosolic redox balance, which brought OxD roGFP to 21.8 ± 8.1% (n = 13; Figures 2A,B,E). Upon recovery of the ΔVo, the roGFP oxidation remained at its oxidized level in 8 out of 13 slices studied, hardly showing any recovery during the remaining ~30 min of recording (Figure 2A). In the other 5 slices, a partial or even near-complete recovery of the SD-related redox changes was observed (Figure 2B). Inspecting the recorded image series revealed that the oxidative wavefront propagated at a velocity of 2.52 ± 0.47 mm/min (n = 13; Figures 2F,G).
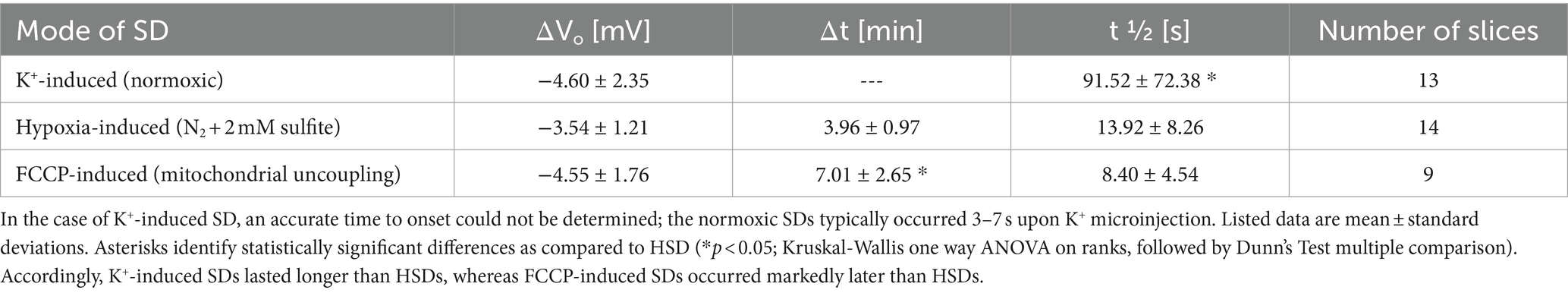
Table 1. Electrical parameters determined for the different types of SD, i.e., amplitude (ΔVo), time to onset (Δt), and duration (t1/2) of the extracellular DC potential deflection.
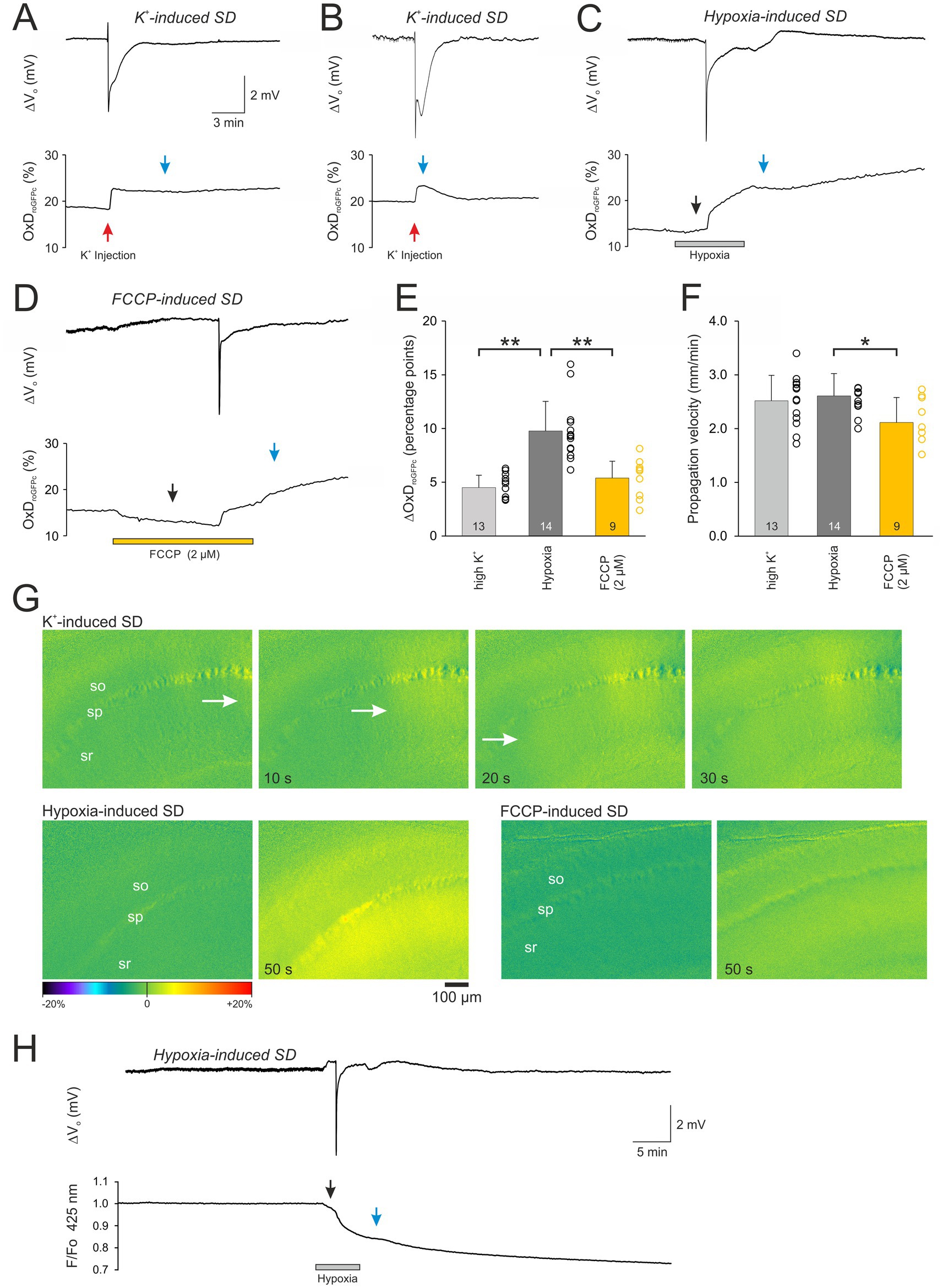
Figure 2. The different modes of SD evoke a pronounced oxidative shift in cellular redox balance. (A,B) Normoxic, K+-induced SD recorded as extracellular DC potential deflection (ΔVo) with the associated redox alterations expressed as degree of roGFPc oxidation (OxDroGFPc). Note the variable reversibility of the redox changes. Red arrows indicate the time point of K+ microinjection, blue arrows the time point at which roGFPc oxidation was quantified. (C) Hypoxia (N2 + 2 mM sulfite)-induced SD with the associated irreversible redox alterations. Note that a moderate roGFPc oxidation started already before SD onset (black arrow). (D) FCCP-induced SD with associated irreversible redox alterations. Administration of FCCP and the resulting mitochondrial uncoupling evoked a noticeable reducing shift before SD occurred (black arrow). (E) Magnitude of the SD-related oxidative changes in the neuronal cytosolic redox balance. Plotted are the changes in the degree of roGFP oxidation (ΔOxDroGFPc) as mean ± standard deviation; the number of slices analyzed is reported for each bar. The dots next to each bar represent the scatter of the underlying data points. Note that most pronounced redox changes occurred during HSD. The extent of roGFPc oxidation was quantified either at the peak or in those conditions not reaching a clear peak, 300 s after SD onset, see blue arrow marks. (F) Propagation velocity of the oxidative wavefront during the different modes of SD. Propagation of SD was lowest in the case of FCCP-induced SDs. Asterisks indicate significantly different changes (* p < 0.05, ** p < 0.01; one way ANOVA and Holm-Šídák comparison versus hypoxic SD). (G) Image series depicting the onset and propagation of the SD-associated redox wavefronts for the conditions of K+-, hypoxia-, and FCCP-induced SDs. The hippocampal layers are indicated (so stratum oriens, sp stratum pyramidale, sr stratum radiatum). Time tags of the respective images indicate the time passed from the first images displayed. Scale bar is identical for all images. The white arrows indicate the propagating wavefront. Images are subtraction images (first image of the time series subtracted from all subsequent images). The displayed changes in roGFPc fluorescence ratios (± 20% range) are displayed in pseudo-colors, with warmer colors indicating a change toward oxidation (i.e., increased roGFPs ratios). (H) Imaging roGFPc fluorescence at its isosbestic point (425 nm excitation) reveals the non-redox-related optical changes during HSD, most of which can be expected to be cell swelling diluting the cytosolic fluorophore concentration. A recovery of the fluorescence decrease did not occur upon reoxygenation.
In the case of HSD, a moderate oxidation started during application of the hypoxic solution already before SD onset, shifting OxDroGFPc from its prehypoxic baseline of 16.4 ± 4.1% to 17.6 ± 3.9% (n = 14, Figure 2C). SD occurred within 3.96 ± 0.97 min of hypoxia and its amplitude averaged −3.5 ± 1.2 mV (n = 14, Table 1). It was paralleled by a marked and more rapid further oxidation to an OxDroGFPc of 26.2 ± 3.6% (n = 14). Whereas the oxidation was more intense than during normoxic SD (Figure 2E), the oxidative wavefront propagation velocity was almost identical (2.61 ± 0.41 mm/min, n = 14; Figures 2F,G). A clear plateau was, however, not reached in the case of HSD; even though wash-out of the hypoxic solution was started 3 min after SD onset, roGFP typically continued to oxidize further for the remainder of the experiment. This continued oxidation obviously indicates a loss of viability, as the O2 scavenger Na2SO3 included in the hypoxia-inducing solution could not be removed from the slices sufficiently fast, hampering a successful re-oxygenation of the vulnerable tissue.
The mitochondrial uncoupler FCCP (2 μM) caused an initial decrease (reduction) of the roGFP1 ratio, shifting OxDroGFPc from its pre-treatment baseline of 15.6 ± 5.3% to 13.1 ± 5.0% (n = 9). This indicates that basal mitochondrial ROS production constantly challenges cytosolic redox balance (Figure 2D). Within 7.0 ± 2.7 min of FCCP treatment the chemically-induced SD occurred, and the ΔVo amplitude averaged −4.6 ± 1.8 mV (n = 9). In parallel a clear roGFP oxidation occurred, shifting OxDroGFPc to 21.0 ± 5.6% (Figure 2D). The magnitude of roGFPc oxidation was similar to normoxic SD, but less intense than during HSD (Figure 2E). The propagation velocity of the oxidative wavefront averaged 2.11 ± 0.47 mm/min (n = 9; Figures 2F,G), being somewhat slower than during hypoxia-and K+- induced SDs. Wash-out of FCCP was started 3 min after SD onset, but the SD-related oxidation did not recover. Instead, roGFPc was oxidized further for the rest of the experiment (Figure 2D), similar to the conditions during HSD.
As SD is accompanied by marked changes in the optical properties of the tissue (intrinsic optical signal, IOS), we also performed experiments in which roGFPc was excited at its isosbestic point of 425 nm (Figure 2H). Induction of hypoxia resulted in a moderate decline of the recorded roGFP fluorescence already before HSD onset, averaging 3.8 ± 0.9% (n = 4) as compared to prehypoxic baseline conditions. Occurrence of HSD was accompanied by a further fluorescence decline, which at the 300 s second time point used for analysis averaged 17.7 ± 2.8% (n = 4). When oxygenation was restored, a recovery was not observed and 40 min upon reoxygenation the fluorescence decline measured 30.0 ± 2.6% (n = 4). As roGFPc was, however, excited ratiometrically for redox analyses, these intrinsic changes in fluorescence intensity – which can be assumed to reflect mostly cell swelling and the associated dilution of cytosolic roGFP content – are minimized by the ratiometric approach.
3.4 Recovery from SDThe irreversible roGFPc oxidation observed during the conditions of hypoxia-and FCCP-induced SDs suggests an impairment of cellular viability. To confirm this assumption, we evoked field excitatory postsynaptic potentials (fEPSPs) by Schaffer-collateral stimulation; these orthodromic stimuli were applied every 30 s for the entire experiment. As expected, O2 withdrawal abolished the evoked responses already before HSD onset. Upon reoxygenation, fEPSPs failed to recover whereas roGFPc continued to become more oxidized (n = 4; Figure 3A). Eliciting fEPSPs during K+-induced SD showed that fEPSPs vanished during the course of SD but then started to recover within a few minutes (n = 3), finally reaching their original amplitude while also the roGFPc oxidation recovered (Figure 3B).
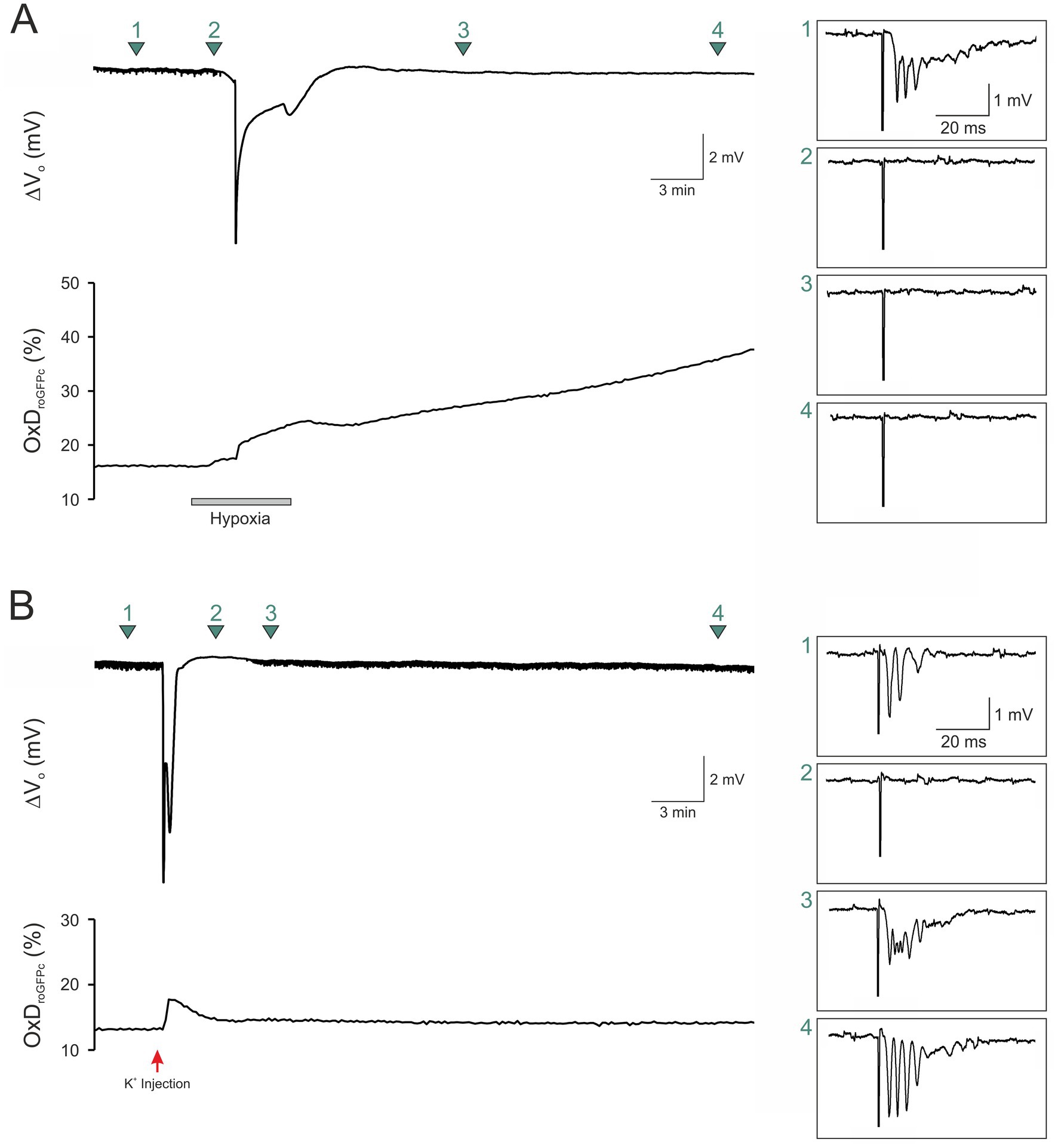
Figure 3. Sustained roGFP oxidation is accompanied by a loss of cellular viability. (A) Eliciting fEPSPs (1.0 mA orthodromic stimuli) revealed a loss of synaptic function during hypoxia even before HSD onset. Upon reoxygenation, neither the roGFPc oxidation nor synapses recovered. Green arrow marks indicate the exact time points at which the displayed fEPSPs were recorded. Stimulation artifacts were truncated to fit the boxes. (B) Normoxic, K+-induced SD also blocked synaptic function, but within minutes, roGFPc oxidation as well as fEPSPs (0.5 mA orthodromic stimuli) fully recovered. Red arrow indicates the time point of K+ microinjection.
3.5 Mechanistic sources of the SD-associated cytosolic oxidationVarious cellular sources generate ROS, thereby challenging cellular redox balance. These include besides mitochondria especially NADPH oxidase and xanthine oxidase (Dröge, 2002; Finkel, 2011). Also, pronounced cellular Ca2+ load may drive cell-endogenous ROS production (Görlach et al., 2015). The following experiments therefore aimed to clarify which of these sources contribute to the SD-related oxidation.
To assess the involvement of cellular Ca2+ influx via voltage-and transmitter-activated Ca2+ channels, slices were pretreated for 15 min with Ca2+-free conditioning ACSF. It should be noted here, that this treatment does not prevent Ca2+ release from intracellular stores, which however only moderately contributes to the massive SD-related cellular Ca2+ load. Ca2+ withdrawal per se did not affect OxDroGFPc baseline conditions (n = 21; see Table 2). However, when K+-induced SDs were induced in the absence of extracellular Ca2+, roGFP oxidation was significantly dampened. The OxDroGFPc rose by only 2.13 ± 0.73 percentage points (n = 9) and more importantly it became fully reversible (Figures 4A,C). Furthermore, the propagation velocity of the oxidative wavefront dropped to 1.74 ± 0.37 mm/min (n = 9; Figure 4D). The oxidative shift during HSD was also significantly decreased in Ca2+-free solutions. The OxDroGFPc increase measured only 4.92 ± 1.47 percentage points (n = 12) and fully recovered upon reoxygenation (Figures 5A,B). In addition, the propagation velocity decreased to 1.88 ± 0.38 mm/min (n = 12; Figure 5C). The electrophysiological parameters of the DC potential shifts during KSD and HSD were not significantly affected in the absence of extracellular Ca2+ (see Table 3).
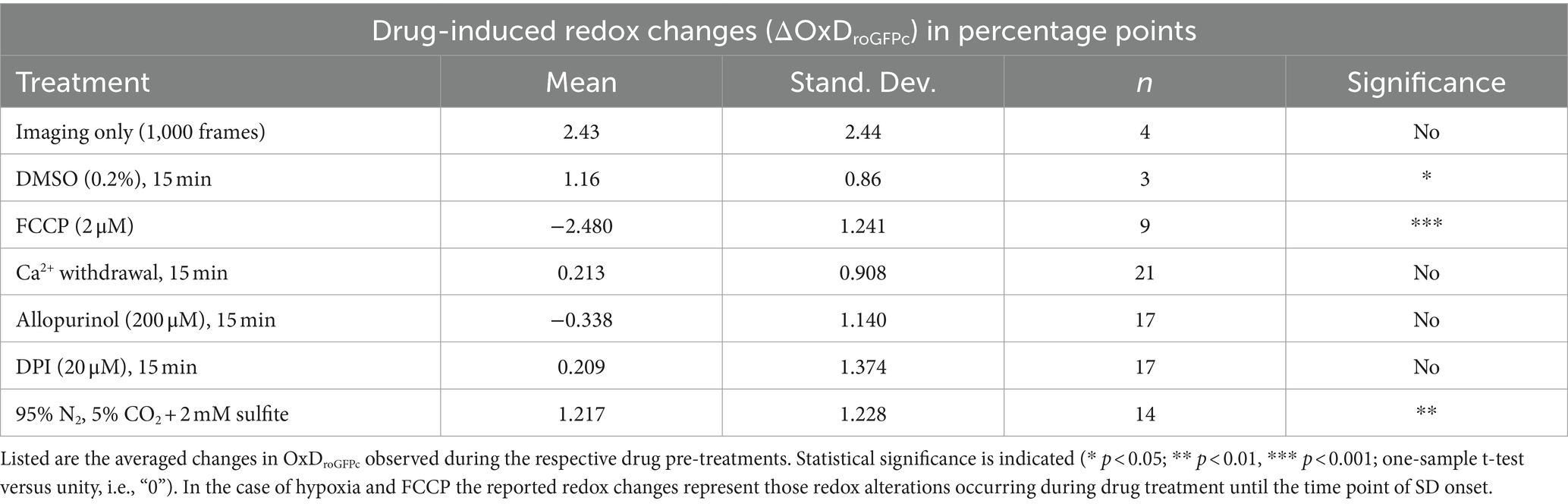
Table 2. Redox alterations during drug application.
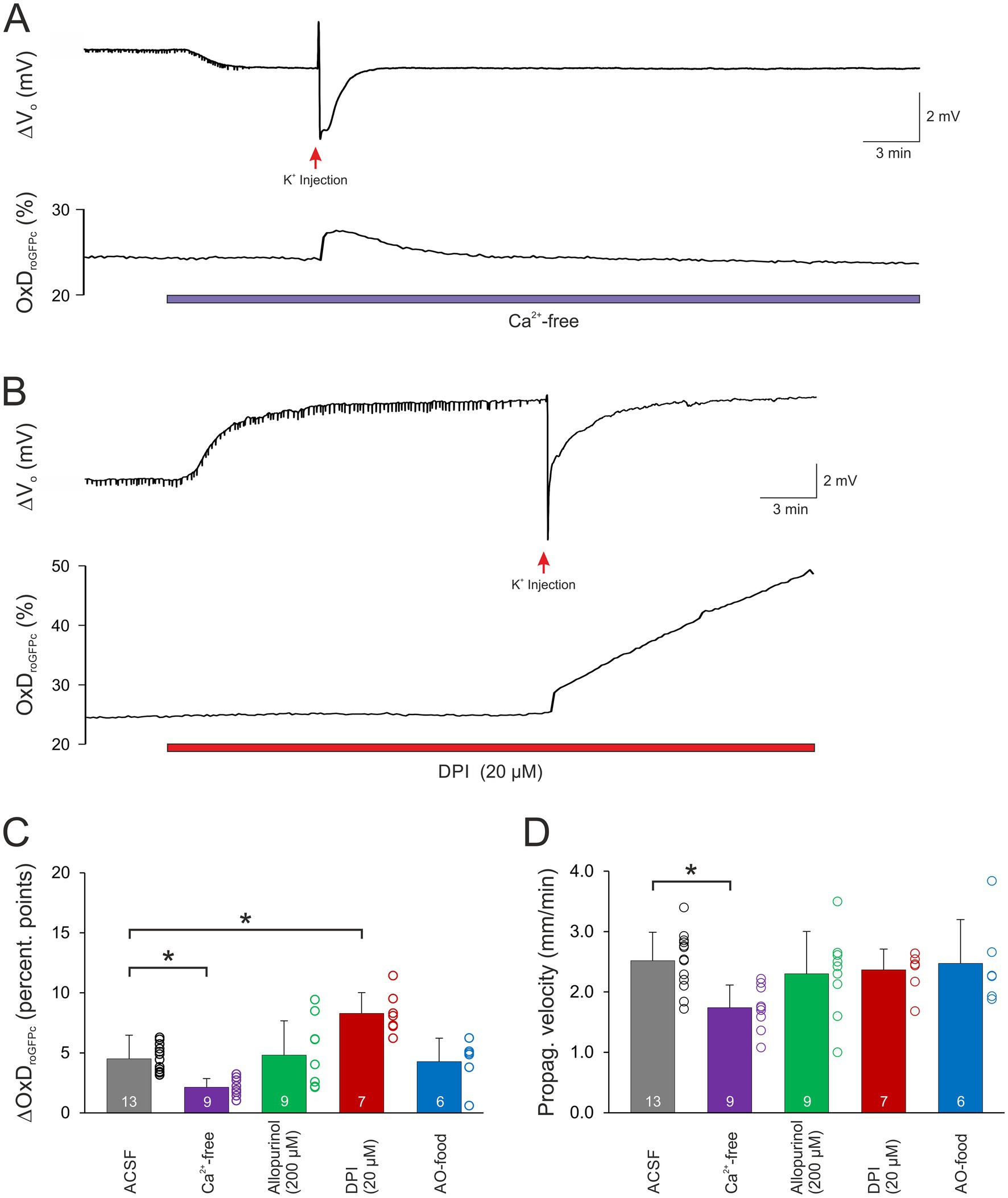
Figure 4. Modulation of normoxic SD-related redox changes. (A) Ca2+ withdrawal dampened the roGFPc oxidation associated with normoxic SD. Furthermore, the oxidative shift became fully reversible. (B) Upon pre-treatment with DPI (20 μM, 15 min), the SD-associated roGFPc oxidation became irreversible. Wash-in of DPI caused a positive shift of the extracellular DC potential, which is due to an offset induced by this compound at the reference electrode; it occurred in all experiments with DPI and averaged 5.3 ± 3.7 mV (n = 17). (C) Magnitude of the normoxic SD-associated oxidative shift, quantified as changes in OxDroGFPc. In Ca2+-free solutions, the oxidative shift was significantly less pronounced, in the presence of DPI it became more intense (*p < 0.05; one way ANOVA on ranks with Dunn’s test multiple comparisons versus ACSF). (D) The propagation velocity of the oxidative wavefront during normoxic SD was not significantly affected by most treatments. Only upon withdrawal of extracellular Ca2+ a slower propagation was observed (*p < 0.05; one way ANOVA and Holm-Šídák multiple comparisons versus ACSF).
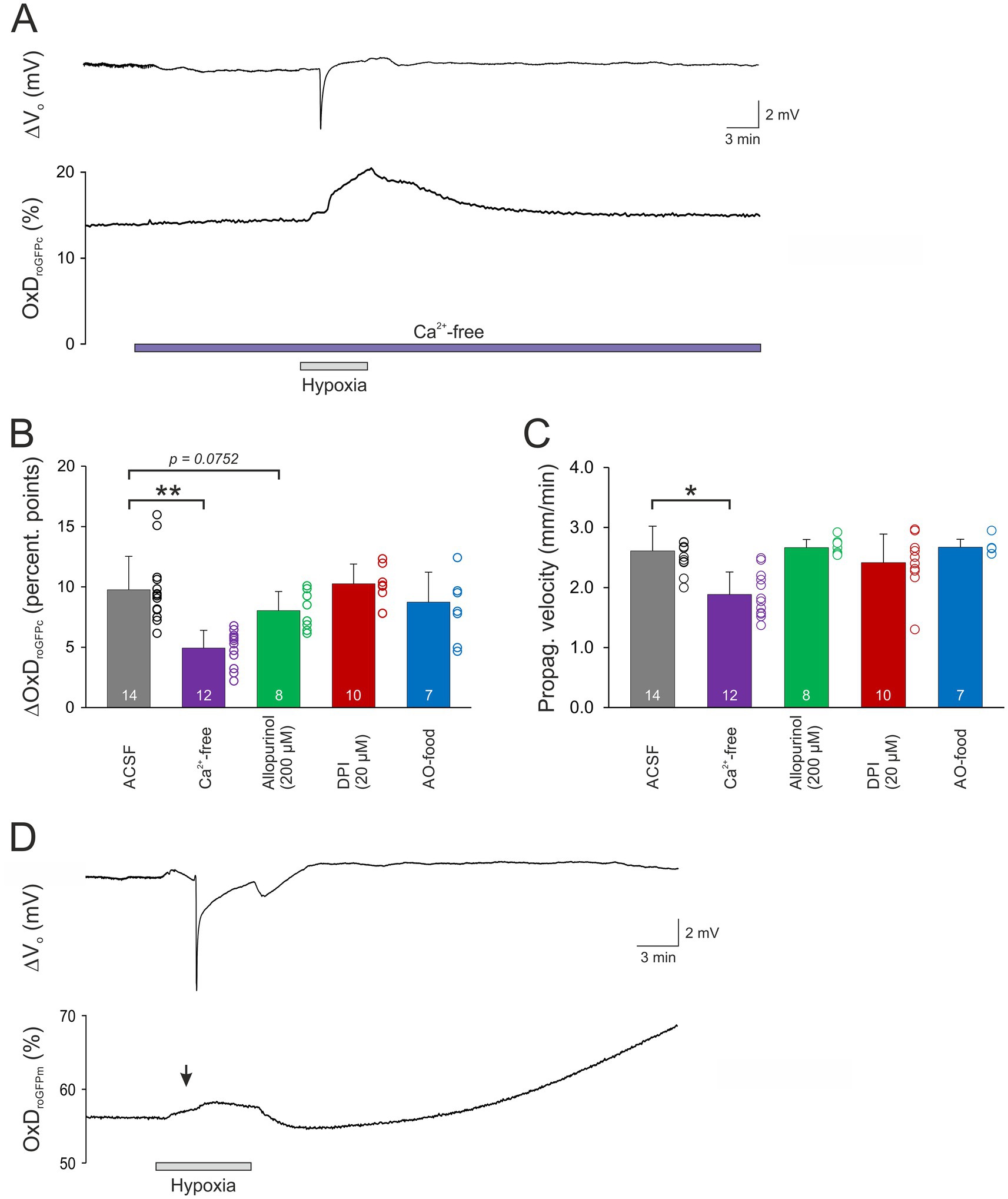
Figure 5. Modulation of HSD-related redox changes. (A) In the absence of extracellular Ca2+ the magnitude of the roGFPc oxidation associated with HSD was significantly dampened and became largely reversible upon reoxygenation. (B) Statistical summary of the redox alterations confirms a significant decrease in Ca2+ free solution and a tendency of dampening upon allopurinol treatment. The number of slices studied is indicated for each group (**p < 0.01; one way ANOVA with Holm-Šídák multiple comparisons versus ACSF). (C) The propagation velocity of the oxidative wavefront was also decreased in Ca2+ free solutions, whereas the other treatments did not mediate any significant changes. The number of slices is reported (*p < 0.05, Kruskal Wallis one way ANOVA on ranks with Dunn’s test multiple comparisons versus ACSF). (D) HSD-induced oxidation of mitochondrial matrix. Mice expressing roGFP in mitochondria (roGFPm mice) revealed that mitochondrial matrix shows markedly less intense redox changes than cytosol. Induction of hypoxia evoked already a moderate oxidation (see black arrow mark). As HSD occurred, this initial oxidation became only slightly more intense and it quickly recovered upon reoxygenation, before mitochondrial matrix then underwent a secondary, slowly progressing oxidation for the remaining duration of the experiment. Note that redox baseline-conditions in mitochondrial matrix are more oxidized than in cytosol.
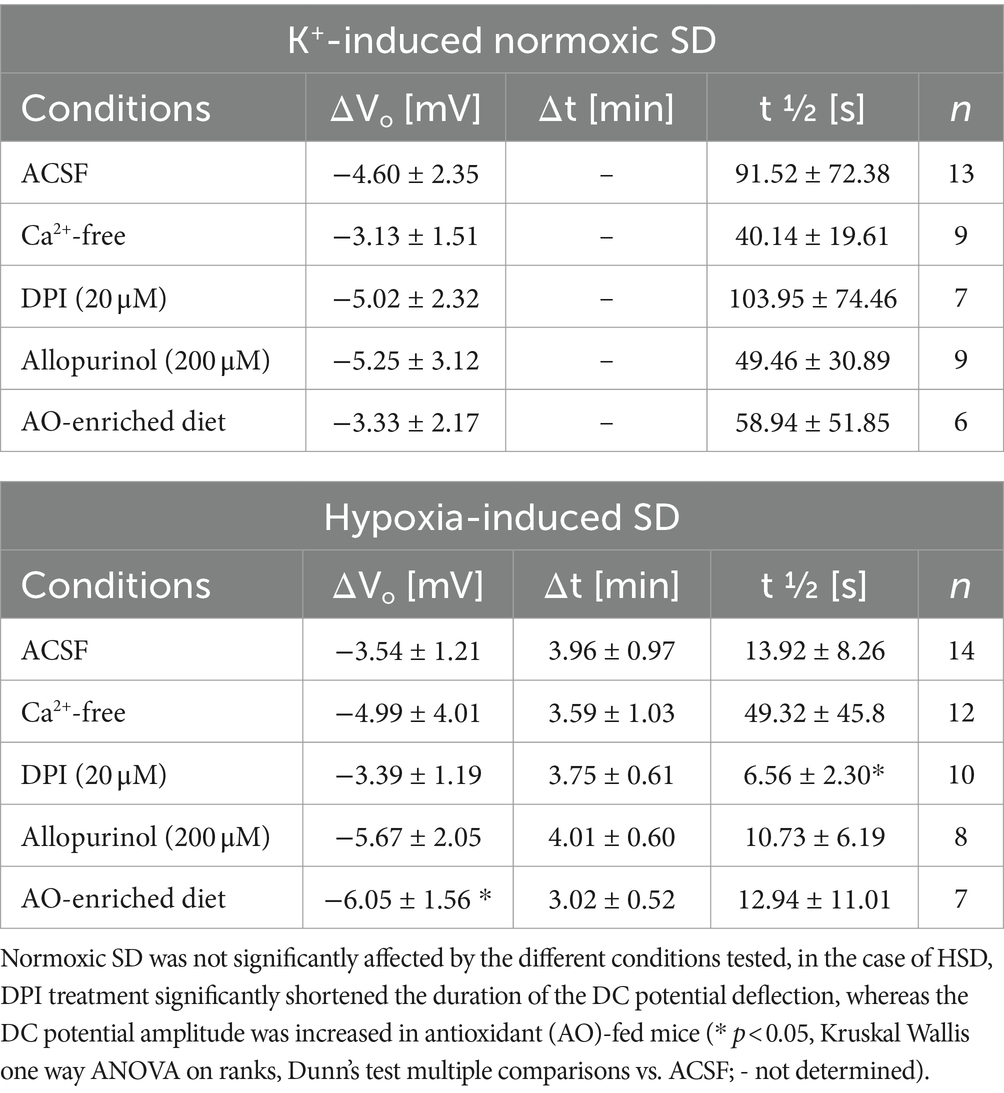
Table 3. Summary of the characteristic parameters of the negative DC potential deflection (amplitude ΔVo; time to onset Δt; duration t ½) that were associated with normoxic and hypoxia-induced SD.
Application of allopurinol, an inhibitor of xanthine oxidase (200 μM, 15 min), did not induce any changes in the resting redox balance of CA1 pyramidal neurons (n = 17 slices, Table 2). Induction of normoxic SD in the presence of allopurinol did not modify the ΔVo parameters (Table 3), the oxidizing shift, OxDroGFPc increased by 4.81 ± 2.85 percentage points, or its propagation velocity (n = 9; Figures 4C,D).
When HSD was evoked in the presence of allopurinol a tendency toward dampened roGFPc oxidation was observed (p = 0.0752, Figure 5B), with OxDroGFPc increasing by 8.04 ± 1.58 percentage points (n = 8). The wavefront propagation velocity, however, remained unaffected (Figure 5C). Neither were the electrical HSD parameters affected.
Pretreating slices with the NADPH inhibitor DPI (20 μM, 15 min) did not evoke any significant changes in redox baseline conditions either (n = 17 slices). When normoxic K+-induced SD was recorded in the presence of DPI, the ΔVo remained unaffected, but the associated redox changes became less reversible, and in part a further slow roGFPc oxidation occurred. Accordingly, the OxDroGFPc changes associated with K+-induced SD were intensified, averaging 8.30 ± 1.72% (n = 7, Figures 4B,C). The propagation velocity of the oxidative wavefront was not affected (Figure 4D). Inducing HSD in the presence of DPI shortened the duration of the ΔVo (Table 3), but did not reveal any obvious effects on OxDroGFPc or the wavefront propagation (Figures 5B,C).
As a more general approach to improve cellular ROS buffering, some mice received an AO-enriched diet (see Material and Methods). In AO-treated mice, the resting redox baselines in CA1 stratum pyramidale corresponded to an OxDroGFPc of 15.6 ± 3.2% (n = 13), which tended to be slightly more reduced than in normally fed mice (17.4 ± 5.8%, n = 98) but the level of significance was not reached (unpaired two-tailed t-test). Evoking a K+-induced SD in these mice did not reveal any significant effects on the electrical or the optical redox parameters of SD (n = 6, Figures 4C,D). Likewise, the roGFPc oxidation during HSD or the wavefront propagation were not significantly affected by AO treatment (Figures 5B,C); the amplitude of the DC potential deflection was slightly increased though (n = 7; Table 3).
3.6 Is SD associated with an oxidation of mitochondria?As O2 withdrawal primarily targets the mitochondria, it remains to be clarified how their redox balance is affected by SD. To solve this question, we used our transgenic roGFPm mice expressing roGFP within mitochondrial matrix (Wagener et al., 2016). For reliable quantitation, we first calibrated roGFPm responses in CA1 stratum pyramidale (Rox = 3.601 ± 0.160, Rred = 1.021 ± 0.033, F470ox/F740red = 0.321 ± 0.017; n = 4). Inducing HSD in these mice revealed a clearly distinct redox pattern (Figure 5D). Showing an OxDroGFPm of 67.3 ± 6.7% (n = 10), baseline redox conditions in mitochondrial matrix were more oxidized than in cytosol. Upon O2 withdrawal, a moderate oxidation of roGFPm was detected before HSD onset. On average, HSD occurred within 2.85 ± 0.73 min of hypoxia and the ΔVo amplitude averaged −4.35 ± 2.20 mV (n = 7). During HSD, only a moderate further oxidation of mitochondrial matrix occurred, and OxDroGFPm was shifted to oxidation by only 1.42 ± 1.50 percentage points (n = 10; range 0.02–5.33). Upon reoxygenation, matrix redox balance quickly recovered to baseline conditions. At the 300 s analysis time point (blue arrow marks in Figure 2) at which the cytosolic oxidation upon HSD was clearly developed, mitochondrial matrix was already indistinguishable from pre-hypoxic baseline conditions. However, several minutes upon reoxygenation, mitochondrial matrix then underwent a delayed oxidative shift continuing for the remaining duration of the experiment (Figure 5D), similar to what we observed in cytosol during HSD.
4 DiscussionHere we present the first assessment of neuronal ROS production and subcellular redox dynamics during K+-, hypoxia-and chemically-induced SD. These experiments became possible based on our redox-indicator mice, which stably express roGFP1 in either cytosol or mitochondrial matrix of excitatory projection neurons (Wagener et al., 2016), as well as an optimized recording chamber developed for combined optical and electrical SD recordings in submerged slices.
4.1 Advanced properties of roGFPAmong the advantages of roGFP is that these sensors are ratiometric by excitation, reliably quantifying cellular redox conditions largely independent of sensor concentrations, cell volume changes and sensor bleaching (Dooley et al., 2004; Hanson et al., 2004). Being fully embedded into cellular thiol redox balance and responding reversibly to oxidizing and reducing stimuli, roGFP reports cellular thiol redox dynamics in real-time. The equilibration of roGFP with the ambient thiol redox conditions is catalyzed by glutaredoxins and thioredoxins (Dooley et al., 2004; Björnberg et al., 2006). As we confirmed earlier, roGFP1 is hardly affected by intracellular Cl- and pH changes (Funke et al., 2011), which is crucial in view of the massive ionic disturbances during metabolic compromise and SD. With these properties it outperforms any synthetic dye such as the fluorescein and rhodamine-derivatives used earlier to detect ROS formation and oxidative stress [see for example (Foster et al., 2006; Can et al., 2017)]. Furthermore, our current experiments exclude any interferences with repeated illumination, DMSO, and autofluorescence of vital tissue (Spitzer et al., 2011). Nevertheless, as demonstrated by isosbestic point-excitation, roGFPc fluorescence is affected (decreased) by the altered optical properties in the SD-invaded tissue. SD is associated with an intrinsic optical signal (IOS), which represents cell volume and morphological changes as well as changes in tissue autofluorescence within the invaded tissue (Andrew et al., 1999; Müller and Somjen, 1999; Müller and Mané, 2010; Sword et al., 2024). This IOS is typically measured as light reflectance, light transmission or as tissue autofluorescence. As demonstrated in Figure 1H, tissue autofluorescence is too weak to markedly contribute in our experiments. Reflected or transmitted light are of no concern as any backscattered excitation (395 and 470 nms) is blocked by the 495 dichroic and 525/50 emission bandpass filter (see Figure 1A) from reaching the CCD camera. So what remains as a potential contribution under our recordings conditions are cell volume changes arising from the SD-related cell swelling. These can, however, be considered to be minimized by the ratiometric approach applied here for roGFP-based redox imaging.
4.2 Spreading depolarization-mediated neuronal oxidationParallel recordings of extracellular DC potentials and neuronal redox conditions confirmed a pronounced roGFPc oxidation during SD. This applied to all types of SD induction tested, i.e., O2 withdrawal, K+ microinjection and mitochondrial uncoupling. The oxidation of roGFPc was strictly linked to the electrical signs of SD, starting together with the ΔVo but then building up more slowly. It represents an oxidizing shift in cytosolic thiol redox balance due to intensified cellular ROS production overwhelming the cell-endogenous redox-buffering systems. The very oxidant species underlying these changes, cannot be specified for sure. Based on the reactivity and lifetimes of various oxidants, most of their cellular signaling is considered to arise from H2O2, as it shows the longest lifetime and hence may diffuse appreciable distances within cells (Pryor, 1986). This view is also supported by the intensified H2O2 levels detected upon K+-induced SD in rat cortex (Viggiano et al., 2011).
Earlier studies taking advantage of lucigenin chemiluminescence to monitor the time course of superoxide formation in rat cortex in vivo obtained a somewhat different picture: K+-induced cortical spreading depression did not induce any detectable superoxide formation, whereas cortical spreading ischemia or transient global ischemia led to a decline in superoxide levels during the ischemic phase, followed by increased superoxide formation during reperfusion (Dirnagl et al., 1995; Dreier et al., 1998). Peri-infarct depolarizations arising during the ischemic period did not affect lucigenin chemiluminescence (Peters et al., 1998). Applying this approach to corticohippocampal brain slices, showed a similar biphasic pattern of dampened superoxide formation during hypoxia and an oxidative burst upon reoxygenation (Dirnagl et al., 1995). To understand these differences, the detailed properties of the two sensors need to be considered. Lucigenin chemiluminescence, a probe for superoxide (Li et al., 1998), reports the very time point of superoxide production, but not the resulting consequences for the cellular/tissue environment. It lacks, however, cellular/compartmental specificity, requires complex reaction pathways, and its reliability has been debated (Faulkner and Fridovich, 1993; Liochev and Fridovich, 1998). In contrast, r
Comments (0)