Background: Whether Inuit in Canada experience disparities in lung cancer survival remains unknown. When requiring investigation and treatment for lung cancer, all residents of Nunavik, the Inuit homeland in Quebec, are sent to the McGill University Health Centre (MUHC), in Montréal. We sought to compare survival among patients with lung cancer at the MUHC, who were residents of Nunavik and Montréal, Quebec, respectively.
Methods: We conducted a retrospective cohort study. Using lung cancer registry data, we identified Nunavik residents with histologically confirmed lung cancer diagnosed between 2005 and 2017. We aimed to match 2 Montréal residents to each Nunavik resident on sex, age, calendar year of diagnosis, and histology (non–small cell lung cancer v. small cell lung cancer). We reviewed medical records for data on additional patient characteristics and treatment, and obtained vital status from a provincial registry. We compared survival using Kaplan–Meier analysis and Cox proportional hazards regression.
Results: We included 95 residents of Nunavik and 185 residents of Montréal. For non–small cell lung cancer, median survival times were 321 (95% confidence interval [CI] 184–626) days for Nunavik (n = 71) and 720 (95% CI 536–1208) days for Montréal residents (n = 141). For small cell lung cancer, median survival times were 190 (95% CI 159–308) days for Nunavik (n = 24) and 270 (95% CI 194–766) days for Montréal residents (n = 44). Adjusting for matching variables, stage, performance status, and comorbidity, Nunavik residents had a higher hazard of death (hazard ratio 1.68, 95% CI 1.17–2.41).
Interpretation: Nunavik residents experience disparities in survival after lung cancer diagnosis. Although studies in other Inuit Nunangat regions are needed, our findings point to an urgent need to ensure that interventions aimed at improving lung cancer survival, including lung cancer screening, are accessible to Inuit Nunangat residents.
Inuit in Canada have shown remarkable resilience in the face of a colonization that included systematic efforts to erase their culture and language and that sustains multiple health disparities compared with non-Indigenous Canadians. Health care services in Inuit Nunangat (the northern portion of Canada that is the traditional Inuit homeland) are characterized by limited accessibility, availability, and acceptability.1–3 The Calls to Action issued by the Truth and Reconciliation Commission of Canada provide the framework for the country to address past and ongoing injustices toward Indigenous Peoples. Health disparities are direct consequences of these injustices, and the Calls to Action include directives for governments and the health sector to identify, track, and eliminate gaps in Indigenous health outcomes.4,5
Inuit in Canada are reported to have the highest incidence of lung cancer in the world.6–8 Lung cancer diagnosis and care pathways are complex and resource intensive, necessitating advanced radiologic and interventional modalities, and multidisciplinary expertise, that are not available in Inuit Nunangat.9,10 It is unacceptable that all steps in the lung cancer diagnostic and management pathways require residents of Inuit Nunangat to travel thousands of kilometres. Whether Inuit Nunangat residents are more likely than other populations in Canada to experience later-stage diagnosis, delays in treatment, and disparities in lung cancer survival remains unknown.
One-quarter of the Inuit Nunangat population reside in Nunavik, the northern third of the province of Quebec. Computed tomography is not available in Nunavik, and only 2 communities have year-round availability of chest radiography. All Nunavik inhabitants receive cancer-related investigations and care at a single centre — the McGill University Health Centre (MUHC) in Montréal, more than 1400 km from Nunavik hospitals, and 1900 km from Nunavik’s most remote village. We sought to compare lung cancer survival between residents of Nunavik and Montréal who received treatment at the MUHC.4
MethodsStudy setting and data sourcesWe undertook a retrospective, matched cohort study. Nunavik has 14 geographically remote villages, and more than 90% of its inhabitants are Inuit. Travel between Nunavik communities and to the rest of Quebec is by air, and access to medical services is limited. The Nunavik Regional Board of Health and Social Services (NRBHSS) is responsible for regional health services. The MUHC, a quaternary care hospital network in Montréal, is the sole institution responsible for providing specialized care to Nunavik residents, including lung cancer diagnosis and treatment, which requires multiple flights and extended stays away from home.
In the registrar-maintained MUHC lung cancer registry, we used postal codes to identify residents of Nunavik and of Montréal newly diagnosed with lung cancer between Jan. 1, 2005, and Dec. 31, 2017. We set 2005 as the start date as there was uncertainty about completeness of lung cancer registry data before this. The end date corresponded to the most recent complete calendar year that we could have included during data collection. Using an automated algorithm (“match” function of the matching package in R),11 we matched each Nunavik patient to 2 Montréal patients on sex and histologic type (non–small cell lung cancer or small cell lung cancer), age (within 4 yr), and year of diagnosis (within 3 yr). We did not match on stage or on subtype of non–small cell lung cancer, so that we could observe differences in distributions of these variables.
After matching, we used a standardized electronic form for medical record review to verify eligibility and collect data on variables not included in the lung cancer registry. We excluded patients missing histologic confirmation. Montréal residents were excluded if the Nunavik resident to whom they were matched had been excluded, if their or their Nunavik match’s lung cancer histology differed from that recorded in the registry, or if they did not receive treatment at the MUHC. We did 1 round of repeat matching, drawing from unselected patients to replace excluded Montréal residents.
VariablesThe variable of interest was geographic residence at time of diagnosis, in either Nunavik or Montréal. We collected lung cancer registry data on age, sex, date of diagnosis, histology, and stage (American Joint Committee on Cancer TNM [tumour, node, metastasis] classification). We used medical records to verify histology and stage, and for data on smoking status, comorbidities (calculating the Charlson Comorbidity Index), Eastern Cooperative Oncology Group (ECOG) Performance Status Scale, treatment decisions, and dates of treatment initiation. The lung cancer registrar updated vital status and dates of death via the provincial health insurance board on Mar. 25, 2019.
Sample sizeBased on NRBHSS cancer reporting for 2001–2010 (268 cancer cases, of which 32% were primary lung cancer), we expected to identify at least 86 Nunavik residents in the lung cancer registry over our study period.12 With 1:2 matching, we estimated 85% power at a 0.05 level of significance to detect a hazard ratio for mortality of 1.5, assuming an unadjusted analysis with no censoring.13
Statistical analysisWe used the Kaplan–Meier approach to estimate survival probability 5 years after diagnosis, stratified by residence and histology. To estimate survival over time from the date of lung cancer diagnosis, we used Kaplan–Meier survival analysis stratified by histology (non–small cell lung cancer v. small cell lung cancer), residence, and stage. We used Cox proportional hazards regression to estimate crude and adjusted hazard ratios (HRs) for mortality between Nunavik and Montréal residents. We verified the proportional hazards assumption using the method of weighted residuals. 14 When deciding which variables to include in our final model, we prioritized addressing confounding over considerations about possible mediation. This meant that if a variable was plausible as a potential confounder of our primary association of interest — between residence and survival — but was also plausible as a mediator, we favoured conceptualizing it as a confounder to be more conservative (in other words, we accepted potential misspecification of mediators as confounders as the resulting bias would attenuate our primary association). We included matching variables in the final multivariable model as doing so reduces bias in matched cohort studies when nonmatching variables are also adjusted for.15 The following variables that had not been matched on were included in the final model as they were considered plausible sources of residual confounding: histologic subtype of non–small cell lung cancer (using a 3-level variable: nonsquamous non–small cell lung cancer as reference, squamous non–small cell lung cancer, and small cell lung cancer); stage (using a 2-level variable: “early” included non–small cell lung cancer stage 1 or 2 or limited small cell lung cancer, and “advanced” included the other disease stages); ECOG performance status; and Charlson Comorbidity Index score. Smoking was not included in the final multivariable model because we thought its impact would be largely mediated through comorbidities for which there were far fewer missing data (we later assessed smoking in a sensitivity analysis; see below). Because stage violated the proportional hazards assumption, we stratified by stage. We used multiple imputation to address missing data (method of chained equations, mice package, using the pool function to combine estimates16 in R) basing the number of imputed data sets on the percentage of incomplete cases.17
In sensitivity analyses, we evaluated the association between residence and mortality in alternative multivariable models that used different groupings for stage and histology, adjusted for specific comorbidities, included smoking status, or accounted for matching by computing robust standard errors.18 In exploratory analyses that did not use imputed data, we compared treatment decisions, rationale for treatment decisions, and time from tissue diagnosis to surgery, first doses of curative-intent radiotherapy, and chemotherapy.
Community contributions to analysisThe NRBHSS was involved at the outset of the study (executive director and the regional director of cancerology, respectively, coauthors M.G. and N.B.). In 2018, we presented interim results from lung cancer registry data to the Inuit-led NRBHSS Board of Directors (representing all 14 communities) who agreed with pursuit of detailed data collection through chart review. After completion of statistical analyses, Puvaqatsianirmut, a newly established Nunavik lung health research steering committee (chaired by coauthor L.W.) contributed to interpretation of findings within the context of Nunavik health care system resourcing. Results and co-interpretations were reviewed with the NRBHSS Board of Directors and regional clinicians before manuscript submission. Nunavimmiut partners contributed to the writing of this report (M.G., L.W., and S.W.-D.).
Ethics approvalThe study was conducted following Tri-Council Policy Statement–2 chapter 9 guidance, and with approval and support of the NRBHSS. The MUHC Research Ethics Board provided research ethics oversight.
ResultsThe 3466 Montréal residents and 98 Nunavik residents in the lung cancer registry were similar with respect to sex and year of diagnosis, whereas Nunavik residents tended to be younger and more likely to have small cell lung cancer (Nunavik, 24.5% v. Montréal, 9.9%; p < 0.01) (Appendix 1, Supplement Table E1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230682/tab-related-content).
After matching, we excluded 33 patients after verifying data in medical records (Figure 1). Because all Nunavik residents listed in the registry had been included at the chart review stage, the 3 subsequently excluded could not be replaced and their 6 Montréal matches were also excluded. For the other 24 excluded patients, we repeated a second round of matching and were able to replace 19. The final cohort consisted of 95 Nunavik residents and 185 Montréal residents. The year of diagnosis ranged from 2005 to 2017 and the distribution of year of diagnosis in the 2 groups was similar.
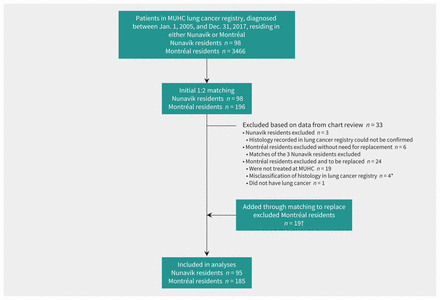
 Figure 1:
Figure 1: Flow diagram for selection of patients in the McGill University Health Centre (MUHC) lung cancer registry to a matched cohort study comparing lung cancer survival between patients residing in Nunavik and in Montréal. *Two Montréal residents with non–small cell lung cancer were excluded after chart review determined that the Nunavik resident to whom they had been matched had small cell lung cancer; 1 Montréal resident was excluded after chart review determined that they were erroneously classified in the registry as having non–small cell lung cancer when in fact they had small cell lung cancer; 1 Montréal resident classified as having small cell lung cancer in the lung cancer registry was excluded after chart review found no histologic confirmation. †We could not replace exclusions from the Nunavik cohort (n = 3, as all Nunavik residents in the registry had already been included), and we did not replace their Montréal matches (n = 6). Hence, there was a total of 24 excluded patients for whom we sought replacement. We added 19 Montréal residents instead of 24 because a replacement meeting matching criteria could not be found (n = 1), and mismatches between Montréal and Nunavik patients on histology were discovered during repeat data verification that took place after the second round of matching (n = 4) and the decision was made to not replace owing to time and funding constraints.
The matched cohorts were similar in age and sex, with similar proportions of non–small cell lung cancer and small cell lung cancer (Table 1). The cohorts differed with respect to non–small cell lung cancer subtypes (among non–small cell lung cancer: squamous cell carcinoma, Nunavik, 64.8% v. Montréal, 29.1%; adenocarcinoma, Nunavik, 25.4% v. Montréal, 62.4%; p < 0.001). Nunavik residents were more likely to be active rather than former smokers at the time of diagnosis (Nunavik, 51.4% v. Montréal, 29.8%). Distributions of stage were similar between the 2 cohorts. Nunavik residents were more likely to have a Charlson Comorbidity Index score greater than 0 (Nunavik, 72.6% v. Montréal, 51.9%), with differences due to greater prevalence of chronic obstructive pulmonary disease (Nunavik, 61/95, 64.2% v. Montréal, 63/185, 34.1%; p < 0.001) and renal insufficiency (Nunavik, 12/95, 12.6% v. Montréal, 9/185, 4.9%; p = 0.04) (Appendix 1, Supplement Table E2). The cohorts had similar ECOG status.
Table 1:Characteristics of the matched cohort
SurvivalFor non–small cell lung cancer, survival probabilities at 5 years after diagnosis were 0.16 (95% confidence interval [CI] 0.08–0.29) for Nunavik residents and 0.31 (95% CI 0.24–0.41) for Montréal residents. For small cell lung cancer, survival probabilities at 5 years after diagnosis were 0.10 (95% CI 0.03–0.36) for Nunavik residents and 0.15 (95% CI 0.06–0.36) for Montréal residents.
Median survival times for non–small cell lung cancer were 321 (95% CI 184–626) days for Nunavik residents and 720 (95% CI 536–1208) days for Montréal residents (p = 0.01) (Figure 2A). Shorter non–small cell lung cancer survival for Nunavik residents was observed across all stages (Appendix 1, Figures E1–E3). Median survival times for small cell lung cancer were 190 (95% CI 159–308) days for Nunavik residents and 270 (95% CI 194–766) days for Montréal residents (p = 0.2) (Figure 2B).
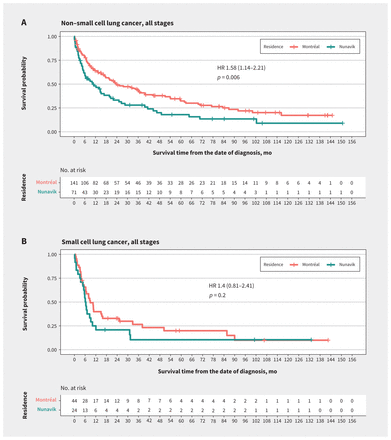
 Figure 2:
Figure 2: Kaplan–Meier survival curves comparing residents of Nunavik and residents of Montréal treated at the McGill University Health Centre. (A) Survival curves of all-stage non–small cell lung cancer patients in the matched cohort. (B) Survival curves of all-stage small cell lung cancer patients in the matched cohort. Note: HR = hazard ratio.
Cox proportional hazards regressionIn the model adjusting for age, sex, year of diagnosis, histologic subtype, stage, ECOG status, and Charlson Comorbidity Index score, residence in Nunavik remained a significant risk factor for mortality (adjusted HR 1.68, 95% CI 1.17–2.41) (Table 2). The association was similar in magnitude and significance in sensitivity analyses (Appendix 1, Supplement Table E3).
Table 2:Results of survival analysis*†
Exploratory analyses of treatment pathwaysAmong patients with non–small cell lung cancer, data on treatment were unavailable for 3 of 71 (4.2%) Nunavik residents and 7 of 141 (5.0%) Montréal residents. Table 3 provides a stage-stratified comparison of the 2 cohorts with respect to treatment approaches among patients with non–small cell lung cancer. For stages 1 and 2 disease, we enumerated reasons why surgery was not pursued. For stages 3 and 4, we enumerated reasons why no treatment was offered. For stages 1 or 2, residents of Nunavik were less likely to be treated surgically (stage 1: 11/18, 61.1% for Nunavik v. 40/45, 88.9% for Montréal; stage 2: 4/7, 57.1% for Nunavik v. 11/14, 78.6% for Montréal). For stages 3 or 4, Nunavik residents were more likely to not receive any cancer treatment (stage 3: 2/16, 12.5% for Nunavik v. 1/27, 3.7% for Montréal; stage 4: 16/25, 64% for Nunavik v. 16/42, 38.1% for Montréal). For small cell lung cancer, proportions of patients receiving treatment were lower for Nunavik residents, but sample sizes were small (Nunavik v. Montréal: limited stage, 10/11, 90.9% v. 19/20, 95.0%; extensive stage, 8/11, 72.7% v. 17/21, 80.9%; Appendix 1, Supplement Table E4).
Table 3:Summary of treatment approaches for non–small cell lung cancer stratified by stage, comparing residents of Nunavik and Montréal
The number of days from histologic diagnosis to the initial curative-intent treatment is shown in Appendix 1, Supplement Table E5 for non–small cell lung cancer and small cell lung cancer, separately. No significant or clinically important differences were noted. Mutation-targeted therapy and immunotherapy were not used in either cohort as the period of analysis preceded their widespread uptake.
InterpretationIn this retrospective matched cohort study, we found that Nunavik residents with lung cancer had shorter survival times than Montréal residents despite similar stage and ECOG performance status at diagnosis. Although small cell lung cancer — which is known to be more aggressive and carry a poorer prognosis than non–small cell lung cancer — was more prevalent in Nunavik, the increased risk of death persisted despite adjusting for multiple potential confounding variables, including histology, stage, comorbidity, and performance status. Exploratory analyses to identify potential treatment-related reasons for the findings were limited by small samples but identified fewer Nunavik residents undergoing surgical resection or, for advanced disease, receiving any treatment.
Two histologic types strongly associated with tobacco smoking — small cell lung cancer and squamous cell non–small cell lung cancer — were more common among Nunavik residents. This is not unexpected given that the prevalence of smoking in Nunavik is much higher than in the rest of Quebec.19,20 The predominance of squamous cell carcinoma has also been reported in other Inuit Nunangat populations21 and means that disparities in survival are likely to worsen in the short term, as mutation-targeted molecular therapies that dramatically improved survival in adenocarcinoma have not been developed for squamous cell carcinoma.22
Distinctions-based approaches should be applied when studying health outcomes of Indigenous populations.23 In the only other Canadian study comparing lung cancer outcomes between residents and nonresidents of an Inuit Nunangat region,24 Asmis and colleagues reported that Baffin Island Inuit had similar survival when compared with controls from the rest of Canada; however, the comparison was not performed directly with patients treated at the same centre, and adjustment for potential confounding variables could not be performed. In our study, where all diagnosis and treatment occurred at the same centre, there is limited potential for bias arising from differences in lung cancer centre resources or expertise.
Overall, the cause for the shorter survival of Nunavik residents is not clear. Histologic subtype of non–small cell lung cancer was associated with both residence and outcome, but the association of residence and survival remained strong despite adjusting for histology. Analyses looking at treatment pathways suggest that patients from Nunavik were less likely to undergo aggressive treatment, but samples were too small to have a clear understanding of why.
We underscore that our results should not be interpreted to conclude that Inuit have a genetic predisposition to worse lung cancer outcomes. Rather, our study observations contextualized with other knowledge about health services and access in Nunavik point to chronic underfunding and underresourcing of Nunavik’s health care services, as well as the lack of Inuit representation in health care provision, as likely upstream determinants of the disparity observed in our study. First, limited resources for chronic respiratory disease prevention, diagnosis, and management render patients from Nunavik at greater risk of lung function impairment precluding surgical lung cancer treatment when indicated.25,26 Chest radiography and spirometry are regularly available in only 2 villages, and Quebec’s smoking cessation support services are not available in Inuktitut, Nunavik’s dominant language. Second, chemotherapy and early palliative care — both known to prolong survival in advanced stages of cancer27,28 — are not available within Nunavik. Our clinical experiences suggest that some Inuit with advanced lung cancer decline chemotherapy because they perceive that its benefit is outweighed by the heavy burden of time away from family and community — however, we emphasize this is not an issue of cultural preference but of structural inequity. That Inuit patients with cancer are more likely than non-Inuit to be in the difficult position of having to choose between life-prolonging treatment and proximity to their support networks contravenes the notion that health care should be equally available to Inuit vis-à-vis other Canadian populations. Third, it is possible that shared decision-making is compromised for patients from Nunavik — as has been shown for residents of other Inuit Nunangat regions29,30 — because of the care trajectory occurring mostly in Montréal without the presence of extended support networks and by cultural and linguistic gaps with health service providers. There are no Inuit navigators with specific lung cancer training within the province of Quebec — in part because such positions do not exist in provincial health care job categories. The underresourcing is not specific to respiratory health; the precarious nature of access to basic health services in Nunavik has been worsening rather than improving,31,32 and becoming ever more evident with population growth.
Several actions can improve lung cancer survival for Nunavik residents. Strengthening of smoking cessation and prevention services, already being pursued by the NRBHSS, will be essential to lessen the population burden of lung cancer and chronic respiratory conditions. Survival overall would improve if more cancers were identified in early stages; hence, lung cancer screening needs to be urgently made accessible and available to this population. There is a need for a Nunavik Inuit–specific lung cancer care plan, designed in partnership with Inuit communities and health leadership, that includes adequate resources for Inuit personnel as navigators,29,33,34 and co-construction of remote oncology services that facilitate access to logistically challenging systemic treatments, including immune checkpoint inhibitors. Interested parties — regional organizations in Nunavik and the health care institutions in Montréal that serve them — should actively lobby provincial and federal governments to provide funding and resources to ensure that health care services widely available in southern Canada can also be accessed within Nunavik, including lung cancer screening. In the bigger picture, observations such as ours should support Nunavik Inuit efforts toward greater self-governance, as increased Inuit decision-making over health care policy and funding will help ensure health services are aligned with the population’s needs.
LimitationsLimitations of our work include that this was a single-centre study. However, the trajectory and logistical challenges of lung cancer care in Nunavik are similar to those experienced in other Inuit Nunangat jurisdictions;35 thus, it is plausible that disparities with non-Inuit populations occur in other locations across Canada.
Another limitation is the proportion of participants missing data on smoking status. Although smoking status is associated with lung cancer outcomes,36 our adjustment for 2 mediators of this association — comorbidities and histologic status — should attenuate bias from missing smoking status.
The sample was too small to pursue further stratified analyses and resulted in low power to detect differences in small cell lung cancer survival.
Medical notes were sometimes not clear in differentiating patient preference from medical recommendations on treatment.
We could not examine rurality as a contributing factor because of selection bias: outside of Nunavik, Quebecers residing in rural locations have access to local hospitals for cancer treatment with only complex cases referred to the MUHC; by contrast, all Nunavik residents receive lung cancer care exclusively at the MUHC. We note that rurality has not consistently been associated with worse cancer outcomes or differential treatment in Canadian studies.37–40
Given that the vast majority of Nunavik residents are Inuit, we could not determine whether the observed disparity was Inuit-specific or residency-specific. However, we note that such a distinction would not affect the recommended actions.
Finally, given the observational nature of our study, there is the potential for confounding from unmeasured factors. We agree with recent calls to ensure that models should not adjust for outcome determinants whose contribution to disparities could be overcome (e.g., through enhanced resources and funding), which we consider to be the case for some unmeasured potential confounders, notably, socioeconomic status and rurality.41
ConclusionWe found that Nunavik residents have a shorter survival after lung cancer diagnosis than Montréal residents receiving treatment at the same centre, even after accounting for multiple potential confounders. A set of explanatory factors was not apparent for the shorter survival via statistical analysis, and further study is needed to better understand potential causes. A contextual analysis with community representatives suggests that addressing broader disparities in health services and resources, increasing Nunavik Inuit self-determination over health care services, establishing Inuit lung cancer navigators, and rapidly deploying culturally safe lung cancer screening may contribute to reductions in survival disparities.
Acknowledgement:The authors thank Glenda Sandy, Eva Quananack, and Elaisa Irniq for their roles on Puvaqatsianirmut in discussing the findings.
FootnotesCompeting interests: Sarah MacIsaac reports fellowship training grants from Janssen Pharmaceuticals and Boehringer Ingelheim, payment or honoraria from Boehringer Ingelheim, participation on a board for Boehringer Ingelheim, and a role on a Canadian Thoracic Society equity, diversity, and inclusion committee. Nathalie Boulanger is director of professional services, Ungava Tulattavik Health Centre. Nicole Ezer reports grants from Canadian Institutes of Health Research (CIHR), MUHC (McGill University Health Centre) Foundation, and Fonds de recherche du Québec – Santé; consulting fees from the GSK Advisory Board for chronic obstructive pulmonary disease (COPD); speaker fees for AstraZeneca family practice clinic teaching on COPD; speaker fees for GSK family practice clinic teaching on spirometry; and advisor roles on a Ministère de la Santé et des Services sociaux committee on lung cancer screening and Institut national d’excellence en santé et services sociaux committee on lung cancer screening. Anne Gonzalez reports a grant from Lung Cancer Canada, and is chair of the Lung Cancer Section, Thoracic Oncology, and Chest Procedures Network, American College of Chest Physicians. Scott Owen reports consulting fees for being on the advisory boards of AstraZeneca, Bristol Myers Squibb (BMS), Roche, Novocure, and Takeda. Carmela Pepe reports advisory board membership and speaker honoraria from AstraZeneca and Merck, speaker honoraria from BMS, and advisory board membership from Takeda. Jonathan Spicer reports grants to his institution from BMS, Merck, AstraZeneca, Roche, Protalix Biotherapeutics, and CLS Therapeutics, and payments from AstraZeneca, Merck, BMS, Roche, Amgen, Pfizer, Xenetic Biosciences, Protalix Biotherapeutics, BMS, and Eisai. Shirley White-Dupuis receives honoraria from the Research Institute of the McGill University Health Centre for expertise and guidance on lung health research projects as a member of Puvaqatsianirmut, and is chair of the board of directors of the Nunavik Regional Board of Health and Social Services. Larry Watt receives honoraria from the Research Institute of the McGill University Health Centre for expertise and guidance on lung health research as a member of Puvaqatsianirmut, and is also executive director of Ungava Tulattavik Health Centre. Minnie Grey is former executive director of Nunavik Regional Board and Health Social Services. Faiz Ahmad Khan reports grants from CIHR, World Health Organization, Fonds de recherche du Québec – Nature et technologies, National Research Council of Canada, and Observatoire international sur les impacts sociétaux de l’IA et du numérique, funded by the Fonds de recherche du Québec. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Faiz Ahmad Khan, Andrea Benedetti, Sophie Camilleri-Bröet, Nathalie Boulanger, Anne Gonzalez, Minnie Grey, and Carmela Pepe contributed to the concept and design. Yue Chen, Faiz Ahmad Khan, Marlene Ahodakin, Sarah MacIsaac, Matthew Young, Maryse Boucher, Luke Wan Jeagal, Sophie Camilleri-Bröet, and Hangjun Wang contributed to the acquisition of data. Yue Chen, Faiz Ahmad Khan, and Andrea Benedetti contributed to the statistical analysis. All authors contributed to the interpretation of data. Faiz Ahmad Khan and Yue Chen drafted the manuscript. All authors contributed to critical revision of the manuscript. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was funded by the Rossy Cancer Network. Faiz Ahmad Khan, Nicole Ezer, and Jonathan Spicer have held salary awards from the Fonds de recherche du Québec – Santé at various points during the conduct of this study. Faiz Ahmad Khan holds the McGill-Institut Nordique du Québec Chair in Respiratory Health and Health Services.
Data sharing: The data set is not publicly available for sharing owing to small annual numbers of lung cancer cases in Nunavik (data sharing could risk breach of confidentiality). Moreover, public sharing has not been discussed with relevant regional stakeholders. The corresponding author may be contacted for clarification.
Accepted January 3, 2024.This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
References↵Systemic discrimination in the provision of healthcare in Inuit Nunangat: a brief discussion paper. Ottawa: Inuit Tapiriit Kanatami; .
↵Access to health services as a social determinant of First Nations, Inuit and Métis health. Prince George (BC): National Collaborating Centre for Indigenous Health; .
↵Truth and Reconciliation Commission of Canada: Calls to action. Winnipeg: Truth and Reconciliation Commission of Canada; :1–20.
↵↵↵↵Inuit cancer control in Canada baseline report.Toronto: Canadian Partnership Against Cancer; :1–55.
↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵↵Taking steps together: a cancer toolkit for providers in Ontario. Ottawa: Tungasuvvingat Inuit; : 1–31.
↵Inuit cancer control in Canada baseline report. Toronto: Canadian Partnership Against Cancer; : 1–55.
↵↵↵↵
Comments (0)