As the third most common gynecological malignancy, ovarian cancer is also the leading cause of death among gynecological cancers (1). In 2012, approximately 239,000 new cases of ovarian cancer and 152,000 deaths due to ovarian cancer were reported worldwide (2). OCCC is a unique subtype within epithelial ovarian cancer and accounts for 5% to 10% of all ovarian cancers in North America, and is more common in East Asia (3). Unlike other types of epithelial ovarian cancer, clear cell carcinoma exhibits distinct epidemiological features as well as clinical and pathological characteristics along with unique gene expression profiles and immune microenvironments.
According to epidemiological data, there are significant differences in the incidence rates among different racial populations. According to a report from the United States, the percentage of ovarian clear cell carcinoma (OCCC) in epithelial ovarian cancer is 4.8% among Caucasians, 3.1% among African Americans, and 11.1% among Asians. In Asia, the percentage of OCCC in Japanese epithelial ovarian cancer has significantly increased from 23.4% in 2002 to 29.1% in 2010, and currently remains above 25% (4, 5). Similar incidence rates have also been observed in Taiwan and Singapore. According to data from Beijing Union Medical College Hospital, the proportion of OCCC is 9.7% (6).
According to organizational studies, the occurrence of ovarian clear cell carcinoma (OCCC) may be related to endometriosis, characterized by the presence of abundant glycogen in the cytoplasm (7). Since Sampson first reported the malignant transformation of ovarian endometriotic cysts in 1925, multiple studies have confirmed a significant correlation between OCCC and ovarian endometriosis (EMs) (8). 50% to 74% of OCCC cases are associated with EMs (with ovarian EMs being the most common) (9). Women with endometriosis have three times higher risk of developing OCCC compared to those without endometriosis (10).
In terms of gene expression OCCC is not strongly associated with family history (11). OCCC shares more similarities with Renal Clear Cell Carcinoma (RCCC) rather than other epithelial ovarian cancer subtypes (12). BRCA1/2 germline mutations are rare in OCCC (2.1% to 6%) (13). Its characteristics include frequent ARID1A somatic mutations (approximately 40-62%), overexpression of MDM2, and upregulation of the PI3K-Akt-mTOR-MAPK signaling axis (approximately 33-51%) (14, 23–25). Additionally, deficiency mismatch repair (dMMR) is relatively more common in OCCC (26).
Given the unique clinical behavior of ovarian clear cell carcinoma (OCCC), we need to gain a better understanding of this rare tumor type. Therefore, the purpose of our study was to evaluate the clinical characteristics and outcomes of OCCC patients, assess the prognostic significance of various clinicopathological features, and provide supporting evidence for the clinical diagnosis and treatment of OCCC.
MethodsPatient and clinical dataThis was a retrospective study conducted on 105 patients diagnosed with primary OCCC at Hubei Cancer Hospital between October 2012 and March 2022. We screened patients according to the prescribed criteria (Figure 1), including patients with complete clinical data and follow-up information. The endpoints selected for analysis included PFS and overall survival (OS). All patients underwent surgical treatment, with histopathology confirming pure ovarian clear cell carcinoma. Exclude patients with concomitant malignancies. Patients who were lost to follow-up were excluded from the survival analysis. Patient information was collected from electronic medical records at our center, including age at diagnosis, menopausal status, reproductive history, FIGO stage, CA-125 levels, ovarian tumor size, bilateral involvement of ovaries, ascitic cytology results, surgical approach used, residual tumor status, pathological diagnosis, presence or absence of coexisting endometriosis, lymph node metastasis, chemotherapy regimen, chemotherapy response rate, PFS, total survival, and disease status at last follow-up visit, and evaluated accordingly. The patients were restaged according to FIGO 2014 staging criteria. Presence of endometriosis was checked according to pathologic report from surgery. Pathological diagnosis according to Sampson and Scott’s criteria, (15, 16), (1) co-existence of OCCC and endometriosis in the same ovary; (2) presence of tissue similar to endometrial stroma around the epithelial glands; (3) exclusion of ovarian metastatic disease; (4) presence of benign endometriosis.
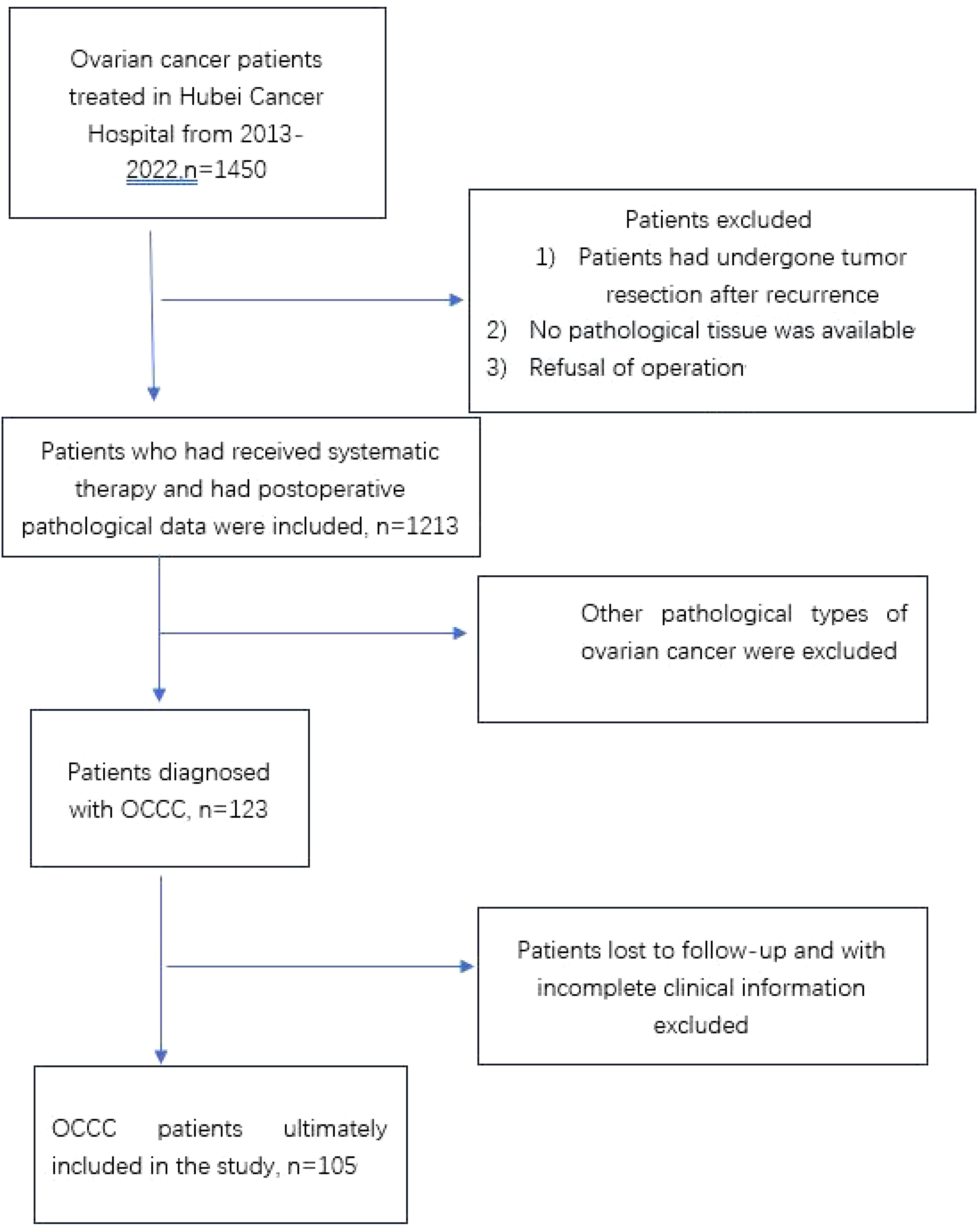
Figure 1. Flowchart of included patients. OCCC, ovarian clear cell carcinoma.
TreatmentsAll patients underwent either open or laparoscopic surgery. Standard surgical procedures included hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic cytoreductive surgery and/or pelvic and para-aortic lymph node dissection. The majority of women underwent complete surgical staging. Except for two cases that refused postoperative chemotherapy, the rest received platinum-based chemotherapy plus paclitaxel regimen for 3-8 cycles according to NCCN guidelines. Based on the residual tumor after the first cytoreductive surgery, tumor residue was categorized as: no residual tumor (RO), residual tumor measuring 0.1-1 cm (R1), and residual tumor measuring >1 cm. The response to chemotherapy was classified as refractory (progression within one month after chemotherapy), resistant (progression within one to six months after chemotherapy), and sensitive (progression after six months of chemotherapy). Patients followed a similar treatment approach throughout the entire treatment phase and strictly adhered to the current NCCN guidelines for ovarian cancer.
Statistical analysisStatistical analysis was performed using SPSS 20.0 for Windows program. The distribution of clinical pathological events was analyzed using chi-square test or Fisher’s exact test. Kaplan-Meier method was used for univariate survival analysis, and Log-rank test was used to compare survival curves. Cox proportional hazards model was used for multivariate analysis to evaluate independent factors affecting survival. A p-value < 0.05 indicated statistical significance.
ResultsWe selected 105 cases of OCCC patients admitted to our hospital from January 2012 to March 2022 as research subjects with more than 12 months of follow-up data available. According to the surgical pathological examination results of ovarian cancer, the patients were divided into 44 cases (41.9%) in the EC-positive group and 61 cases (58.1%) in the EC-negative group. Independent sample t-tests were used to analyze age differences between the two groups statistically; however, no significant difference was found in age distribution between them (P>0.05). Chi-square tests were conducted to analyze menopausal status and whether there was residual disease after surgery among other relevant clinical indicators between both groups statistically; it revealed that there were significant differences in menopausal status distribution as well as referral after incomplete surgery in the another hospital between them (P<0.05).The incidence of menopause in the EC-negative group was higher than that in the EC-positive group, while the rate of referral after incomplete surgery in the another hospital was lower in the EC-negative group compared to the EC-positive group (see Table 1).
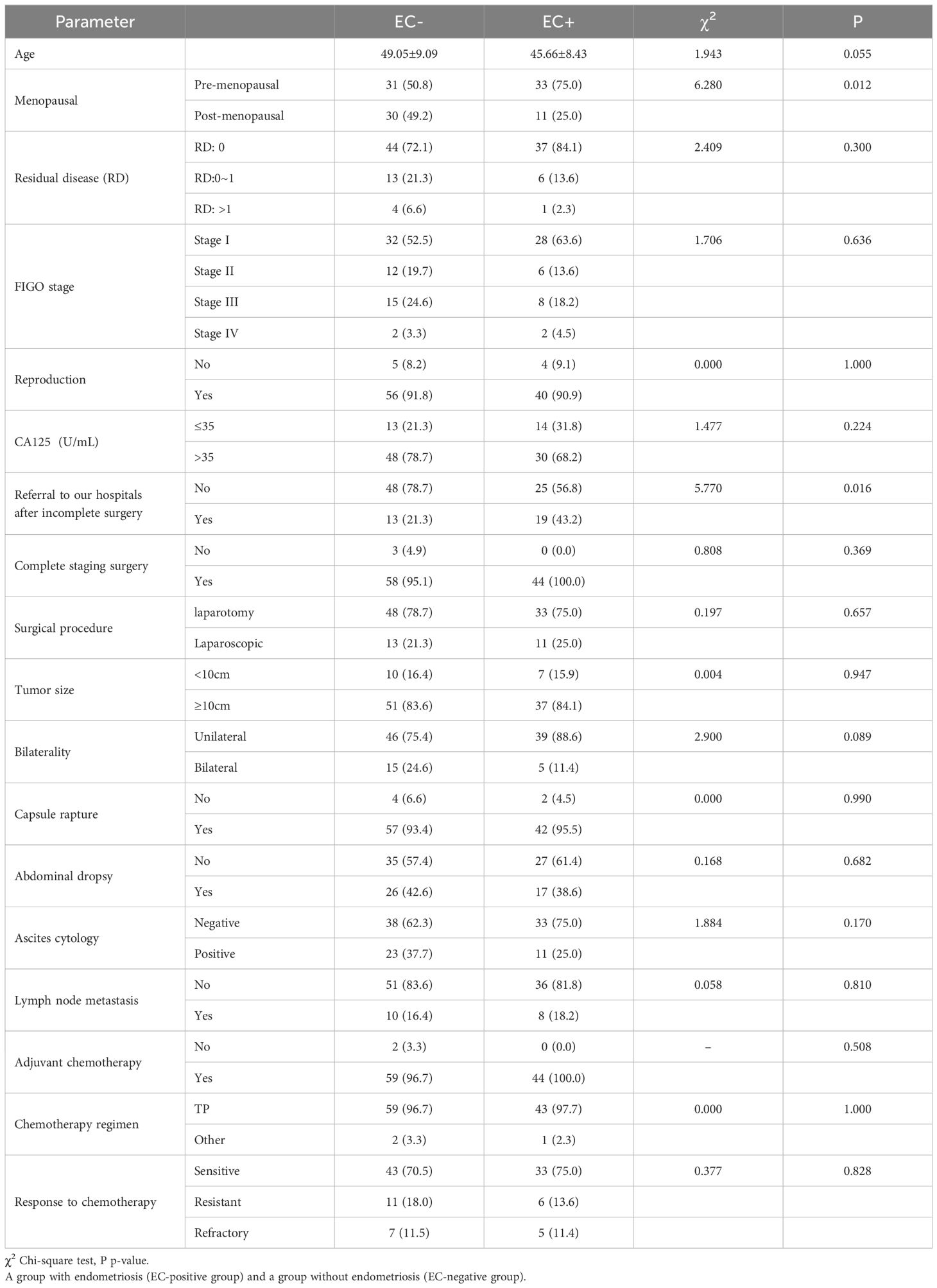
Table 1. Presents the analysis of clinical indicators in patients with ovarian clear cell carcinoma in the EC-positive (EC+) group and the EC-negative (EC-) group.
The asymptomatic survival period, recurrence status, time of death, and survival status of the patients separately, and based on the patients’ recurrence and survival status, the patients were divided into a recurrence group with 59 cases (56.2%) and a non-recurrence group with 46 cases (43.8%), as well as a survival group with 68 cases (64.8%) and a death group with 37 cases (35.2%).
Statistical analysis using univariate Log-Rank test was conducted to examine clinical factors influencing patient recurrence; results indicated that surgical residue, staging, CA125, site of invasion, ascites, cytology of ascitic fluid, lymph node metastasis, chemotherapy effect were associated with patient recurrence outcome and time to recurrence (P<0.10). Cox multivariate survival analysis was performed on statistically significant variables from the univariate analysis. The results indicated that the efficacy of chemotherapy cycles was independent predictors for patient recurrence (P<0.05). Patients who were resistant chemotherapy had a greater risk of recurrence (HR=8.070, 95% C.I.: 4.183-15.569), as shown in Table 2.
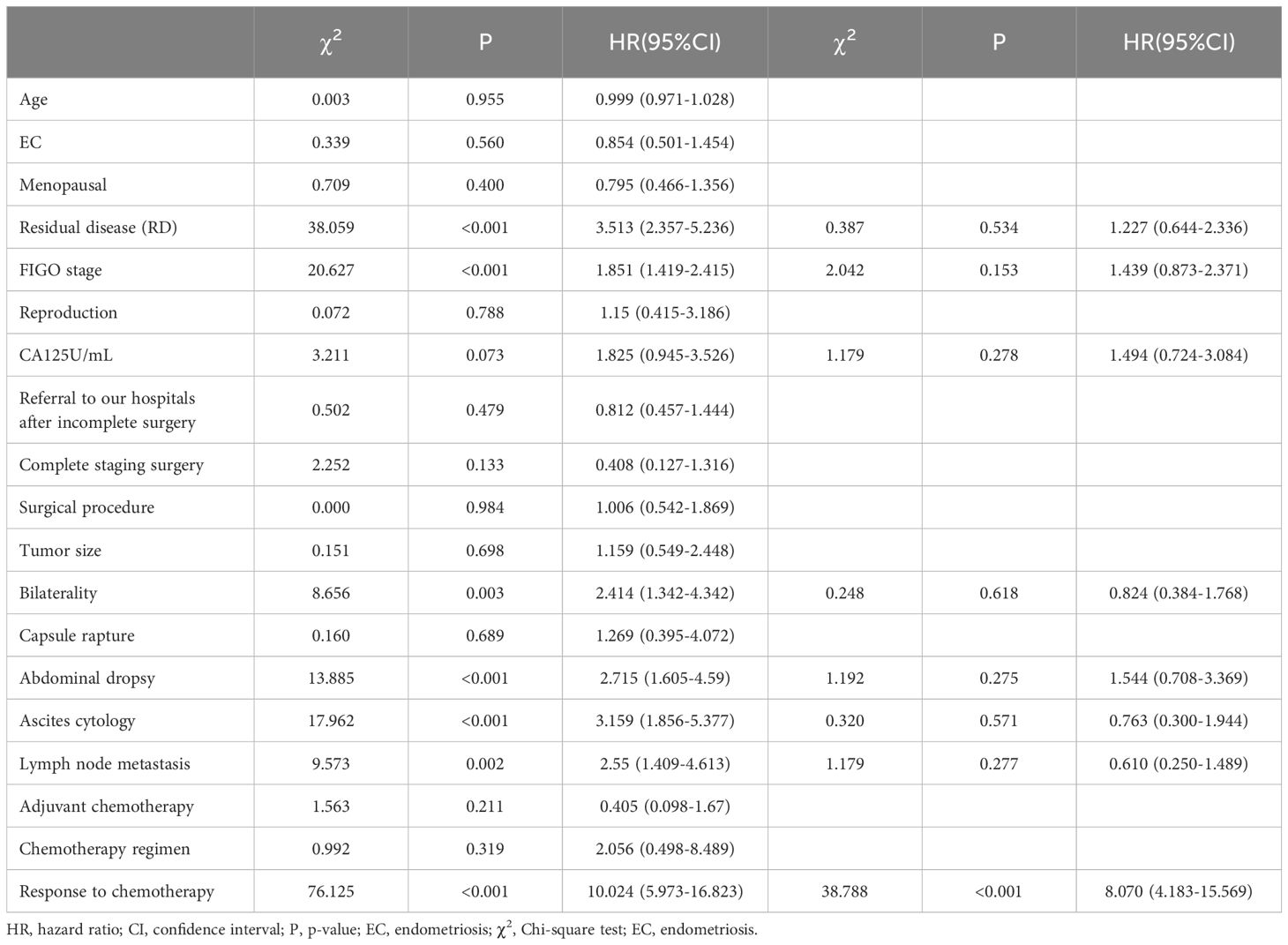
Table 2. Single-factor and multi-factor analysis of factors affecting patient relapse.
Kaplan-Meier curves were plotted to analyze the survival rates for patients with positive EC status versus negative EC status in terms of their recurrences. According to the survival curve analysis, there was no statistical difference in recurrence status and time between patients with positive EC status and negative EC status (P=0.552). The median time to recurrence for patients with positive EC status was 27 months (95% C.I.: 0-97.67), while it was 34 months for patients with negative EC status (95% C.I.: 0-71.09), as shown in Table 3 and Figure 2A.
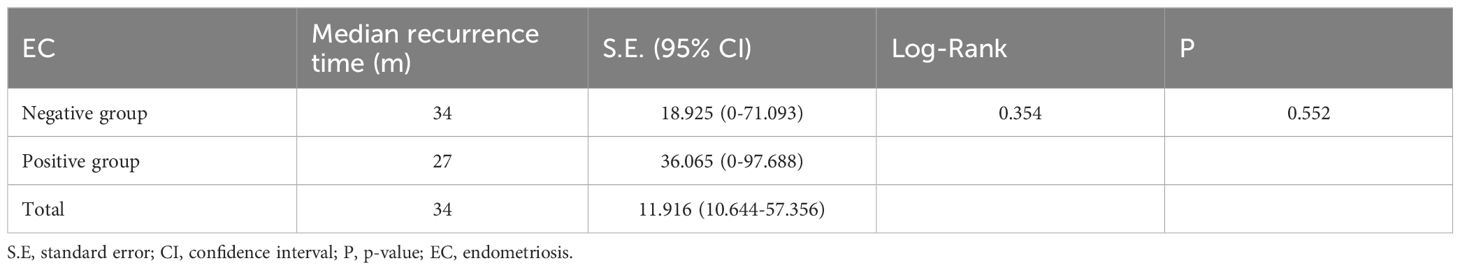
Table 3. Recurrence status and time analysis in EC-positive group and EC-negative group.
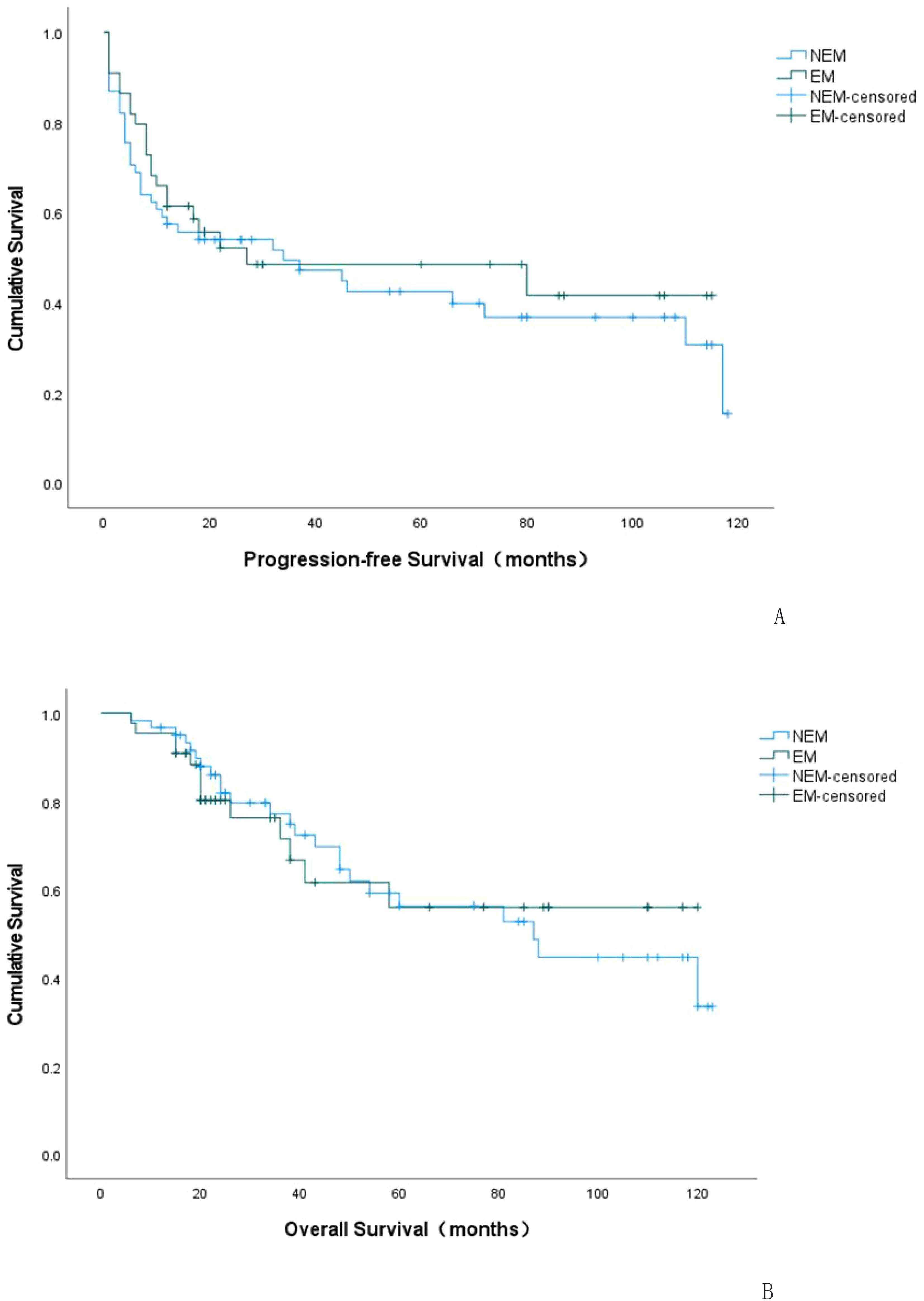
Figure 2. K-M curve of progression-free survival (PFS) in EC-positive group and EC-negative group (A). K-M curve of overall survival (OS) in EC-positive group and EC-negative group (B). NEM: EC-negative group; EM: EC-positive group.
Univariate Log-Rank tests were conducted to statistically analyze clinical factors affecting patient survival rates. The results showed that surgical residue, staging, site invasion, ascites, cytology of ascitic fluid, lymph node metastasis, chemotherapy effect were associated with patient survival outcomes and survival time (P<0.10), which are predictive factors affecting patient survival. Cox multivariate survival analysis was conducted to further analyze the statistically significant variables identified in the univariate analysis. The results indicated that staging and chemotherapy efficacy were independent predictive factors for patient recurrence (P<0.05). Patients with stage 3 or 4 had a higher risk of death compared to those with stage 1 or 2 (HR=2.137, 95% C.I.: 1.068-4.276), while patients resistant or not controlled by chemotherapy had a greater risk of death (HR=5.596, 95% C.I.: 2.942-10.643). Please refer to Table 4 for details.
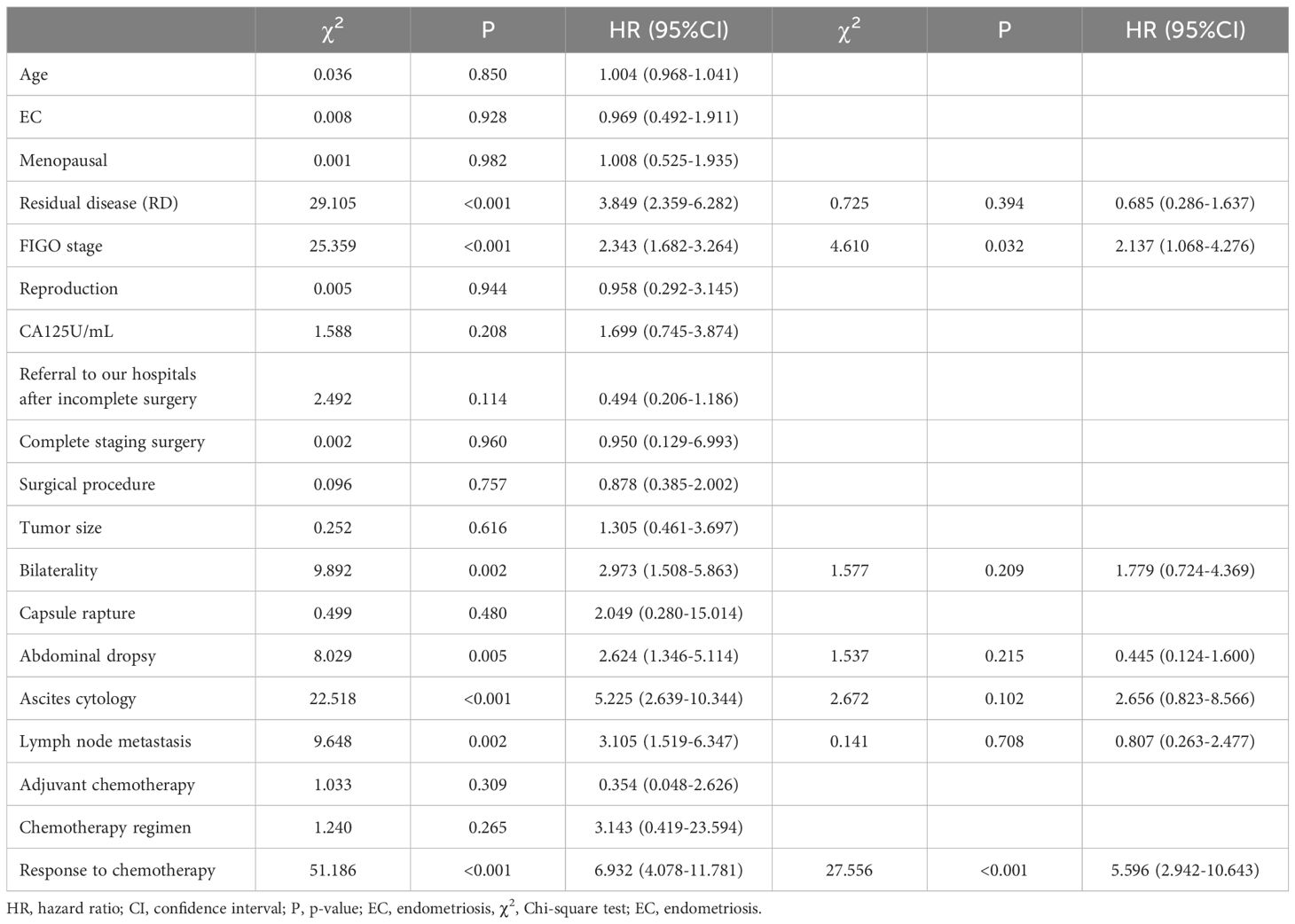
Table 4. Univariate and multivariate analysis of factors influencing patient survival.
Kaplan-Meier curves were plotted to depict the survival outcomes between EC-positive group and EC-negative group in terms of overall survival rate among patients with endometriosis-associated OCCC. According to the analysis based on these curves, there was no statistical difference observed in terms of overall survival rate and time between EC-positive group and EC-negative group (P=0.928). The median overall survival period for EC-positive group was estimated at 80.177 months (95% C.I.:62.817-97.537), while it was calculated as79.204 months (95%C.I.:66.386~92.022)for EC-negative group. Please refer to Table 5 and Figure 2B for details.
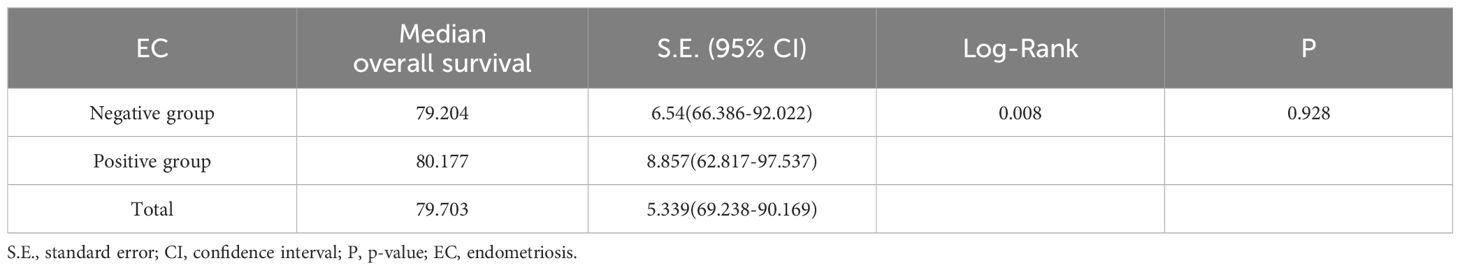
Table 5. Survival status and time analysis in EC-positive group and EC- negative group.
After stratifying selected independent predictors, the effects of endometriosis on PFS and OS were also analyzed (see Table 6). The conclusion was that the two cohorts had no statistical significance in PFS after stratification for chemotherapeutic effect (Chemotherapy sensitivity: p-value=0.8354; Chemotherapy resistance: p-value=0.5275) or FIGO stage in OS after stratification (Stage I: p-value=0.8172; Stage II-IV: p-value=0.1276) or chemotherapeutic effect (Chemotherapy sensitivity: p-value =0.6425; Chemotherapy resistance: p-value=0.1349).
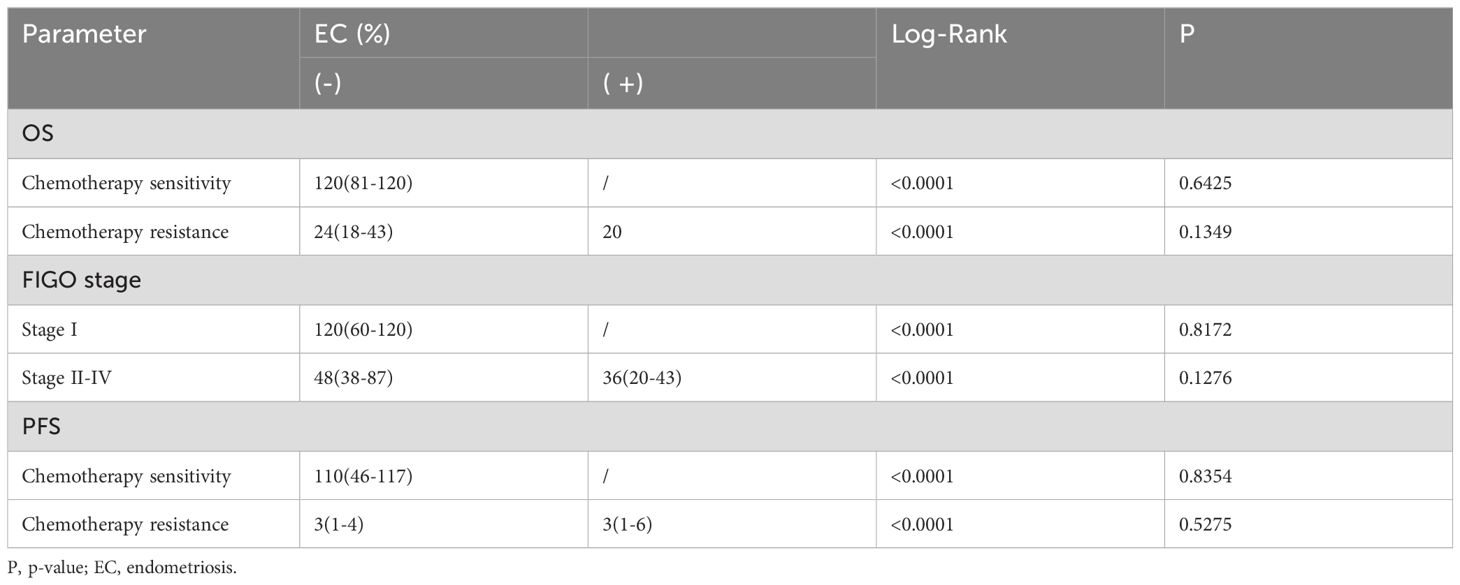
Table 6. Survival analysis after stratification for independent predictors.
DiscussionThe relationship between endometriosis and OCCC has been extensively studied since Sampson first described EAOC in 1925, with multiple studies evaluating the incidence of endometriosis-related OCCC (17). Evidence suggests that women with endometriosis have a total ovarian cancer risk that is 1.2-1.8 times higher than that of the general population (18). Patients with such history are three times more likely to develop OCCC compared to those without it (19). A large-scale epidemiological study on over ninety-nine thousand women with endometriosis found an increased incidence rate of ovarian cancer among them (20). Herein, we examined 105 OCCC patients and investigated the effect of endometriosis as an independent prognostic factor. In this cohort, all patients were Han Chinese women, and approximately 42% of OCCC patients were endometriosis positive. After univariate, multifactorial, and stratified analysis of several confounding factors, we found that endometriosis had no significant impact on prognosis.
OCCC is a special type of EOC pathology characterized by unique clinical and molecular features (22, 27). However, not all women with OCCC have concomitant endometriosis at the time of diagnosis, further demonstrating that endometriosis-free OCCC and endometriosis-related OCCC seem to develop through different pathways. Although the association between ovarian endometriosis and OCCC has been established, the exact underlying molecular transformation mechanisms remain unclear (21). Some scholars believe that bcl-2 and p53 proteins may be related to the malignant transformation of endometrial cysts (42), and it is recommended to establish standards for identifying and monitoring risk factors in women with endometriosis, and to seek risk-reducing drug and surgical treatment options in these women (43).
Currently it is widely believed that patients with Endo-related OCCCs tend to be younger when diagnosed (28, 29); however our findings contradict those reports since our data shows no significant difference in age between groups (P<0.05) but rather an average 4-year younger age at onset for EC-positive group compared with EC-negative group. The reasons for this deviation may be due to limited pathological sampling after surgery where some patients who had concurrent internal heterotopic lesions were not clearly documented; or pathologists may overlook internal heterotopic lesions once they diagnose ovarian cancer or miss them due technical differences; Rapidly growing cancer cells may also destroy the original endometriotic lesions, thereby eliminating histological evidence of endometriosis. Due to conflicting data, further large-scale studies are needed to support the aforementioned viewpoint.
In addition, the single factor analysis in this study found that positive ascites cytology affected patients’ recurrence and survival time, which caused us to think about the current surgery for ovarian cancer. Currently there is no high-level evidence in evidence-based medicine to suggest that minimally invasive surgery has adverse effects (30, 31). Therefore, some clinicians choose laparoscopic surgery for ovarian cancer. In this study, a univariate analysis was used to statistically analyze the clinical factors related to patient recurrence and survival. The results showed that ascites and positive cytology of ascitic fluid were associated with patient recurrence and survival time, making them predictive factors affecting patient survival (P<0.001). However, in clinical practice, patients with endometriosis often present with significant symptoms such as dysmenorrhea and seek repeated medical treatment. Therefore, for early-stage clear cell carcinoma combined with endometriosis patients, a considerable number of physicians may initially diagnose it as benign conditions such as ovarian chocolate cysts and recommend laparoscopic exploration followed by removal of the affected ovary tumor for definitive diagnosis. Studies have shown that endometriosis leads to an average size of 15cm for OCCC ovarian masses which are predominantly multilocular and contain solid component (32, 33). For larger ovarian tumors accompanied by intrauterine adhesions due to endometriosis (34), there is a high risk of cyst rupture during laparoscopic surgery (35) leading to clinical stage upgrading and positive cytology of ascitic fluid which affects patient prognosis. Additionally, during laparoscopy procedures, instruments repeatedly entering contaminated incisions generate airflow and smoke from electrocoagulation or electrosurgery promoting dissemination and implantation of tumor cells; During laparoscopic surgery, filling the abdominal cavity with CO2 gas can cause diffuse damage to the entire abdominal cavity and facilitate the peritoneal dissemination of tumor cells (36–38). The head-down, feet-up position during laparoscopic surgery, once the tumor ruptures, can easily lead to tumor dissemination in the entire abdominal and pelvic cavities, including the upper abdomen.
Moreover, OCCC has a low response to chemotherapy and exhibits some degree of drug resistance (39), thereby increasing the likelihood of tumor recurrence. Therefore, in practical work, careful selection of suitable patients is necessary for laparoscopic surgery to avoid iatrogenic tumor dissemination. Larger-scale studies are needed to validate the aforementioned observations regarding tumor rupture caused by laparoscopic and open surgeries in patients with stage IC1 OCCC.
Given the limited effectiveness of late-stage OCCC chemotherapy, the question of whether early-stage OCCC patients truly benefit from adjuvant chemotherapy has always been controversial. Multiple retrospective studies have shown that patients with advanced ovarian clear cell carcinoma (OCCC) have limited benefits from adjuvant chemotherapy, and there is no statistically significant difference in disease-free survival (DFS) and overall survival (OS) between patients who received chemotherapy and those who did not (41). However, there are also studies supporting the benefit of chemotherapy in early-stage OCCC patients. An analysis of 2,072 stage OCCC patients from the US National Cancer Database (NCDB) showed that those who received adjuvant chemotherapy had a higher 5-year OS than those who did not receive adjuvant chemotherapy(89.2% vs 86.2%, P<0.001) (40). After controlling for factors such as disease stage, age, race, hospital type, and comorbidities, adjuvant chemotherapy was associated with better OS. In this study, all patients were from the same medical center, so the treatment strategy was similar and the majority of patients received chemotherapy with a combination of paclitaxel and platinum drugs. We found no difference in chemotherapy efficacy between the two groups. However, through multivariate survival analysis, we discovered that the chemotherapy effect was an independent predictor of patient recurrence and survival outcomes (P<0.05). The risk of recurrence and death is higher in chemotherapy resistant patients compared to chemotherapy sensitive patients (HR= 5.596, 95%C.I.: 2.942 ~10.643).
In this study, we compiled comprehensive data analysis and reliable follow-up records from patients treated at our medical center with a unified clinical treatment approach. The study reveals the clinical characteristics, treatment features, and prognosis of ovarian clear cell carcinoma (OCCC) in China. Most patients were diagnosed at an early stage and received postoperative chemotherapy regardless of staging. Our findings indicate that endometriosis is not an independent prognostic factor for OCCC women. However, these findings are limited by the number of included patients and the retrospective design of the study, which may introduce selection bias. Therefore, considering the unique biological characteristics of OCCC and its increasing incidence rate, especially in East Asia, larger-scale well-designed prospective studies are still needed to establish a stronger connection between endometriosis and OCCC.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Ethics Committee Office of Hubei Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because This study is retrospective, so informed consent is not required.
Author contributionsYHu: Data curation, Writing – review & editing. YHe: Data curation, Writing – original draft. BC: Data curation, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. The author claims to have received financial support in the course of researching, writing and/or publishing this article. This study was supported by the special Research Fund of Biomedical Center of Hubei Cancer Hospital (grant number: 2022SWZX28).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Qin N, Jiang G, Zhang X, Sun D, Liu M. The effect of nutrition intervention with oral nutritional supplements on ovarian cancer patients undergoing chemotherapy. Front Nutr. (2021) 8:685967. doi: 10.3389/fnut.2021.685967
PubMed Abstract | Crossref Full Text | Google Scholar
2. Stewart B, Wild C. Cancer by organ site: cancers of the female genital organs. In: World Cancer Report 2014. IARC, Lyon, France (2014). p. 465–81.
3. Anglesio MS, Carey MS, Köbel M, Mackay H, Guntsman D. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol. (2011) 121:407–15. doi: 10.1016/j.ygyno.2011.01.005
PubMed Abstract | Crossref Full Text | Google Scholar
4. Anglesio MS, Carey MS, Kobel M, Mackay H, Guntsman D. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell symposium. Gynecol Oncol. (2011) 121:407–15. doi: 10.1016/j.ygyno.2011.01.005
PubMed Abstract | Crossref Full Text | Google Scholar
5. Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO-JSOG joint study. Gynecol Oncol. (2019) 153:589–96. doi: 10.1016/j.ygyno.2019.03.243
PubMed Abstract | Crossref Full Text | Google Scholar
6. Wang S, Qiu L, Lang JH, Keng S, Xin YJ, Fang HH, et al. Clinical analysis of ovarian epithelial carcinoma with coexisting pelvic endometriosis. Am J Obstet Gynecol. (2013) 208:413.e1–5. doi: 10.1016/j.ajog.2012.12.004
PubMed Abstract | Crossref Full Text | Google Scholar
7. VandenBussche C, Crothers B, Fader A, Jackson A, Li Z, Zhao C. Special considerations for peritoneal washings. In: Chandra A, Crothers B, Kurtycz D, Schmitt F, editors. The International System for Serous Fluid Cytopathology. Springer, Cham (2020). doi: 10.1007/978-3-030-53908-5_9
Crossref Full Text | Google Scholar
9. WHO Classification of Tumours Editorial Board. WHO classification of tumours.Female genital tumours. 5th Edition Vol. 65. . Lyon: IARC Press (2020).
10. Zhu CC, Xu ZH, Zhang TJ, Qian L, Xiao W, Wei H, et al. Updates of pathogenesis, diagnostic and therapeutic perspectives for ovarian clear cell carcinoma. J Cancer. (2021) 12:2295–316. doi: 10.7150/jca.53395
PubMed Abstract | Crossref Full Text | Google Scholar
11. Heinzelmann J, Henning B, Sanjmyatav J, Posorski N, Steiner T, Wunderlich H, et al. Specific miRNA signatures are associated with metastasis and poor prognosis in clear cell renal cell carcinoma. World J Urol. (2011) 29:367–73. doi: 10.1007/s00345-010-0633-4
PubMed Abstract | Crossref Full Text | Google Scholar
12. Nguyen JMV, Vicus D, Nofech-Mozes S, Gien LT, Bernardini MQ, Rouzbahman M, et al. Risk of second Malignancy in patients with ovarian clear cell carcinoma. Int J Gynecol Cancer. (2021) 31:545–52. doi: 10.1136/ijgc-2020-001946
PubMed Abstract | Crossref Full Text | Google Scholar
13. Takayuki E, Daisuke A, Kana H, Masahisa J, Junzo K, Nobuhiro T, et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectionaL approach to Ovarian cancer geneTic TEsting of BRC (CHARLOTTE). Int J Gynecol Cancer. (2019) 29:1043–9. doi: 10.1136/ijgc-2019-000384
PubMed Abstract | Crossref Full Text | Google Scholar
15. Samson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. (1925) 10:1–72. doi: 10.1001/archsurg.1925.01120100007001
Crossref Full Text | Google Scholar
16. Scott RB. Malignant changes in endometriosis. Obstet Gynecol. (1953) 2:283–9.
17. Tranoulis A, Buruiana FH, Gupta B, Kwong A, Lakhiani A, Yap J, et al. Friend or foe? The prognostic role of endometriosis in women with clear cell ovarian carcinoma. A UK population-based cohort study. Arch Gynecol Obstet. (2022) 305:1279–89. doi: 10.1007/s00404-021-06191-8
PubMed Abstract | Crossref Full Text | Google Scholar
20. Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer SK, Graubard BI, et al. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev. (2005) 14:2929–35. doi: 10.1158/1055-9965.EPI-05-0394
PubMed Abstract | Crossref Full Text | Google Scholar
21. Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. (2016) 152:R63–78. doi: 10.1530/REP-16-0052
PubMed Abstract | Crossref Full Text | Google Scholar
22. Shin HY, Yang W, Chay DB, Lee EJ, Chung JY, Kim HS, et al. Tetraspanin 1 promotes endometriosis leading to ovarian clear cell carcinoma. Mol Oncol. (2021) 15:987–1004. doi: 10.1002/1878-0261.12884
PubMed Abstract | Crossref Full Text | Google Scholar
23. Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecologic Oncol. (2018) 151:381–9. doi: 10.1016/j.ygyno.2018.09.001
PubMed Abstract | Crossref Full Text | Google Scholar
24. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. New Engl J Med. (2010) 363:1532–43. doi: 10.1056/NEJMoa1008433
PubMed Abstract | Crossref Full Text | Google Scholar
25. Itamochi H, Oishi T, Oumi N, Takeuchi S, Yoshihara K, Mikami M, et al. Whole-genome sequencing revealed novel prognostic biomarkers and promising targets for therapy of ovarian clear cell carcinoma. Br J cancer. (2017) 117:717–24. doi: 10.1038/bjc.2017.228
PubMed Abstract | Crossref Full Text | Google Scholar
26. Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Modern Pathol. (2017) 30:1622–32. doi: 10.1038/modpathol.2017.67
PubMed Abstract | Crossref Full Text | Google Scholar
27. Gadducci A, Multinu F, Stefania CS, Carinelli S, Ghioni M, Aletti GD. Clear cell carcinoma of the ovary: epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol Oncol. (2021) 162:741–50. doi: 10.1016/j.ygyno.2021.06.033
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zhao T, Shao Y, Liu Y, Wang X, Guan L, Lu Y. Endometriosis does not confer improved prognosis in ovarian clear cell carcinoma: a retrospective study at a single institute. J Ovarian Res. (2018) 11:53. doi: 10.1186/s13048-018-0425-9
PubMed Abstract | Crossref Full Text | Google Scholar
29. Ju UC, Kang WD, Kim SM. The effect of concurrent endometriosis on the prognosis of women with ovarian clear cell or endometrioid carcinoma. Int J Gynaecol Obstet. (2019) 146:177–83. doi: 10.1002/ijgo.2019.146.issue-2
Crossref Full Text | Google Scholar
30. Morgan RJ Jr, Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Behbakht K, Chen LM, et al. Ovarian cancer, version 1.2016, NCCN clinical practice guidelines in oncology. Natl Compr Canc Netw. (2016) 14:1134–63. doi: 10.6004/jnccn.2016.0122
PubMed Abstract | Crossref Full Text | Google Scholar
31. Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. (2021) 10:61–85. doi: 10.1002/ijgo.v155.s1
PubMed Abstract | Crossref Full Text | Google Scholar
32. Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinomaof the ovary. Cancer. (2000) 88:2584–9. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5
Crossref Full Text | Google Scholar
33. Cohen JG, Prendergast E, Geddings JE, Walts AE, Agadjanian H, Hisada Y, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. (2017) 146:146–52. doi: 10.1016/j.ygyno.2017.04.021
PubMed Abstract | Crossref Full Text | Google Scholar
34. Hao M, Zhao WH, Wang YH. Correlation between pelvic adhesions and pain symptoms of endometriosis. Zhonghua Fu Chan Ke Za Zhi. (2009) 44:333–6.
PubMed Abstract | Google Scholar
35. Chikazawa K, Imai K, Wang L, Kuwata T, Konno R. A cystectomic technique with low risk of rupture for women with benign ovarian cyst. J Obstet Gynaecol. (2021) 41:459–61. doi: 10.1080/01443615.2020.1755622
PubMed Abstract | Crossref Full Text | Google Scholar
36. Canis M, Rabischong B, Botchorishvili R, Tamburro S, Wattiez A, Mage G, et al. Risk of spread of ovarian cancer after laparoscopic surgery. Curr Opin Obstet Gynecol. (2001) 13:9–14. doi: 10.1097/00001703-200102000-00002
PubMed Abstract | Crossref Full Text | Google Scholar
38. Brundell S, Tucker K, Texler M, Brown B, Chatterton B, Hewett PJ. Variables in the spread of tumor cells to trocars and port sites during operative laparoscopy. Surg Endosc. (2002) 16:1413–9
Comments (0)