It is possible to speculate that the non-linear path of evolution and its violent history, may have led to the development of brains that favored protection and recovery from brain injuries. To name a few, human brains are protected by the cranium, underneath which are three core layers of protective membranes, in addition to being immersed in cerebrospinal fluid capable of absorbing impact. Indeed, recovery has been found through neuroplasticity and neurogenesis, which appear to be far more advanced than previously believed. At some point however, there was a trade-off, the advancement of brain structure and function, for a brain more susceptible to damage through impact. This adaptation functioned because it was predicated on the utility of complex cognitive abilities to offset and/or treat the consequences of Traumatic Brain Injuries (TBI). One way this is expressed in modern day is the development of neuropsychological assessments in service of evaluating, predicting, and improving the outcome of a TBI.
While TBIs were likely around since the dawn of humankind, it is also likely that our brains did not evolve to withstand the impact of a car crash. Nonetheless, when viewing incidence rates, it is clear that comparable injuries our ancestors might have sustained (e.g., from falls, sports, or violence) continues to be a major problem. Worldwide, traumatic brain injuries (TBI) constitute one of the leading causes of injury-related deaths and disability (Maas et al., 2022). TBIs are responsible for ~30% of all injury-related deaths in the United States and are a leading cause of mortality and disability (Kaur and Sharma, 2018). Closed-head injuries (CHI) account for about 75% of TBIs, while penetrating head injuries (PHI) account for around 25% (CDC, 2016). There were ~223,125 TBI-related hospitalizations in 2019 and 64,362 TBI-related deaths in 2020 (CDC, 2022). TBI is the leading cause of death and disability in people younger than age 35 in the US (Popescu et al., 2015). Falls lead to nearly half of the TBI-related hospitalizations and are now the leading cause of TBI, overtaking road traffic accidents (Roozenbeek et al., 2013). Firearm-related suicide is the most common cause of TBI related deaths in the US. In the United States, around 1.7 million people suffer TBI, with older adolescents (ages 15–19 years) and older adults (ages 65 years and older) among the most likely to sustain a TBI (Georges and Das, 2023).
Traumatic Brain Injury (TBI) refers to penetrating, blunt, or acceleration/deceleration force-derived craniocerebral injury. TBI often results in cognitive deficits in memory, attention, processing speed, word finding, planning, and problem-solving. From a behavioral perspective, difficulties such as lack of initiative, irritability, and poor temper control may be present. Somatic symptoms may include headaches, dizziness, fatigue, sleep disturbance, poor balance, and coordination. TBI can also result in psychological symptoms (e.g., anxiety and depression). These difficulties usually resolve to some degree, but could persist in many cases, even decades after injury. Thus, ongoing assessment and tailored interventions are crucial for effectively managing TBI. Neuropsychological assessments are essential for identifying deficits and understanding the extent of functional loss. These assessments predict outcomes and guide treatment, aiming to improve patients' functional abilities while mitigating further cognitive decline. The specific characteristics of a TBI, including the nature and extent of the damage, help determine the type of neuropsychological deficits that may arise. Understanding these characteristics will assist in distinguishing between different forms of head injuries and their impacts.
Types of traumatic brain injuriesHead injuries can be classified into two broad categories: closed head injury (CHI) and penetrating head injury (PHI) (Kaur and Sharma, 2018). CHI is more common and complicated than PHI; over 75% of all brain injuries are CHIs. CHI occurs when an external force impacts the skull, causing damage to the brain without penetrating the skull. Swift forward or backward movement and shaking of the brain inside the cranium are common causes of this type of damage, which results in hemorrhage and the tearing of brain tissue and blood vessels (Vieira et al., 2016). CHIs can affect various areas of the skull, including the frontal bone, temporal bone, parietal bone, and occipital bone, depending on the specific circumstances of the injury (Jeyaraj, 2019). Paradoxically, while the cranium is meant to protect the brain, in certain circumstances, parts of it may cause damage. One such area is the crista galli, a protruding triangular surface rising from the ethmoid bone that plays a role in attaching the dura mater (one of the protective membranes mentioned earlier).
On the other hand, PHI occurs when a foreign object, such as a bullet or a sharp projectile, penetrates the skull and directly damages the brain tissue. It is important to note that PHI can cause significant damage to the brain tissue, as the object can cause both primary and secondary injuries to the brain. Primary and secondary injuries are two distinct phases of TBI that can cause damage to the brain tissue (Ng and Lee, 2019). Primary injury occurs immediately after the traumatic event, caused by the direct physical forces applied to the brain tissue. Secondary injury occurs after the primary injury and can be caused by various processes, including inflammation, oxidative stress, excitotoxicity, and others. These processes may worsen the symptoms of the initial injury and inflict more harm on the brain tissue. Secondary injuries can occur over a period of hours or days after the initial trauma and can contribute significantly to the long-term effects of TBI.
CHI can range in severity from mild concussions to severe TBI. In mild cases, the individual may experience symptoms such as headaches, dizziness, and confusion but may not lose consciousness. Severe CHI can result in long-term neurological deficits and disability. Symptoms of closed head injuries can include loss of consciousness, memory loss, difficulty concentrating, seizures, and changes in personality or behavior.
In contrast, PHI is typically more severe and life-threatening than CHI. The severity of a PHI depends on the location and extent of the damage caused by the foreign object. In general, PHIs result in more localized brain damage, whereas CHIs may result in diffuse damage to the brain. PHIs may thus yield a neurocognitive profile with more targeted deficits. This may however, be complicated by hemorrhaging, infection, and swelling, further damaging the brain tissue. Treatment for penetrating head injuries often involves surgical removal of the foreign object, followed by intensive medical care to manage the resulting brain damage and complications. Recognizing the distinction between closed and penetrating head injuries provides context regarding the diverse nature of TBI and their specific effects on brain regions, with these varying impacts directly influencing cognitive and functional outcomes.
Multiple levels of analysisThe short and long-term sequelae of PHIs and CHIs depend on severity and may be analyzed on multiple levels. Linear and rotational acceleration of the brain can result in mild TBI (mTBI) if a significant amount of force is applied. Blennow et al. (2016) have shown that in these instances, the lower sulci located in the frontal, parietal, and temporal lobes receive higher levels of stress and strain induced by TBI. Cortices and white matter tracts also receive the brunt of the damage induced by mTBI. White matter tracts, which send neuronal signals to nearby neurons and are located in subcortical regions, corpus callosum, fornices, and cerebellum, are more prone to damage upon the initial impact of mTBI.
One of the most common symptoms caused by TBI is cerebral edema. This symptom occurs after the injury and is triggered by an inflammatory response (Arulsamy et al., 2018). Cortical swelling is typically increased in the prefrontal and temporal cortices (Linden et al., 2019; Dall'Acqua et al., 2017). Prolonged and sufficient damage from TBI has the potential to induce an inflammatory response (Bigler, 2013; Johnson et al., 2013) with acute swelling potentially leading to chronic secondary injuries (Ma et al., 2016). That is why, when swelling is severe enough, a portion of the skull may be removed (i.e., craniectomy) to enable the inflammatory processes to take their course, and naturally subside without risking further damage to the brain. Compared with controls, once swelling has subsided, many individuals diagnosed with TBI exhibit reduced brain volume in the temporal, hippocampal, and frontal regions (Bigler, 2013).
Upon impact, axonal shearing may occur near the primary site of injury (Govindarajan et al., 2016). Occurring in areas of the brain initially injured, axonal shearing has been identified as a precursor to the buildup of beta-amyloid plaques, apoptosis, and oxidative stress (Ma et al., 2016). Damage to these areas plays a large role in the symptoms that are experienced. TBI has been shown to produce emotional deficits, challenges with working memory, and other executive dysfunctions. The hippocampus has been correlated to memory related processes and aids in the regulation of emotions. Damage to this area caused by TBI can result in decreased memory capacity and emotional functioning. However, studies have shown that promoting neurogenesis within this region can reduce negative symptoms (Peng and Bonaguidi, 2018).
Moderate to severe TBI significantly reduces cortical thickness (Vedung et al., 2022). Differences in cortical thickness in acute and chronic stages of TBI demonstrate how an injury in the frontal-temporal region correlates to neurodegeneration across the hemispheres. For example, a study was conducted comparing the cortical thickness of mTBI patients where a baseline of decreased cortical thickness was established compared to healthy controls (Govindarajan et al., 2016). Research conducted by Govindarajan et al. (2016) demonstrated that cortical thinning is associated with mTBI primarily in the frontal, temporal, and parietal regions. Follow up examinations of cortical thickness in these subjects revealed the thinning had spread to include some areas of the insula and cingulate cortex (Govindarajan et al., 2016). Research has shown that cortical thickness decreases in adolescents who tested positive for TBI in the prefrontal cortex (Linden et al., 2019). Vedung et al. (2022) established a correlation between a decrease in cortical thickness and an increase in TBI symptom severity, in particular, individuals with moderate to severe TBI exhibit increases in cortical thinning compared with control groups. Within their study, Govindarajan et al. (2016) provided treatment for an mTBI subgroup, participants did not show a significant reduction in cortical thickness after obtaining treatment.
Mild TBI also commonly decreases. However, in some instances, sporadic increases in cortical thickness have been shown to occur post-mTBI, but the increase is not significant compared to cortical thinning (Govindarajan et al., 2016). Individuals who have worse outcomes long term, over 3 months, after the initial injury have lower volumes of gray and white matter and increased cortical thickness compared with healthy controls (Dall'Acqua et al., 2017). The subsequent increase of cortical thickness in mTBI patients has not been well-established, but it could indicate an increase in swelling and trauma.
White and gray matter are largely affected by TBI (Vedung et al., 2022; Dall'Acqua et al., 2017). A decrease in white and gray matter volume inhibits homeostasis as these regions are responsible for neuronal communication and processing. The continuous degeneration of white and gray matter is also found in the neuropathology of neurodegenerative diseases suggesting that secondary injuries of TBI correlate to those diseases (Jang et al., 2017). Jang et al. (2017) found a relationship between the degeneration of white and gray matter tracts and Alzheimer's, Subcortical Vascular Dementia, and mixed dementia, with the highest level of white matter degeneration occurring in Subcortical Vascular Dementia and mixed dementia. The varied impact of TBI on specific brain areas results in diverse experiences and outcomes, with differences observed in cortical thickness, white and gray volume, and the manifestation of secondary injuries.
Findings on how TBIs may impact cortical thickness, white and gray matter, major lobes of the brain, the hemispheres and particular regions may orient the clinician toward specific functions that may be anticipated to be impacted. Yet, real-time dysfunction may be more accurately depicted by a network approach, analyzing neurodynamic imbalances between networks within the brain. Recognizing how TBI disrupts these interconnected networks informs the injury's impact on both localized brain functions and broader cognitive processes.
Neural networks and TBIsWhile there are many neural networks, there are three of particular interest, the Default Mode Network (DMN), the Salience Network (SN) and the Central Executive Network (CEN). The DMN is a network related to mind wandering, autobiographical recall, prospection, self referential processing, and social cognition. The SN determines the significance or salience of external or internal stimuli in any particular moment. It also acts as a toggle between the DMN and CEN. The CEN is a network dedicated to goal-directed tasks and executive functioning. Mutual inhibition typifies the relationship between the DMN and CEN (Chan, 2021). These three networks are typically working together in neurodynamic balance, as the individual shifts their focus toward the external world to focus on a task (CEN) or pauses to reflect on themselves and an interaction that occurred (DMN). In TBIs, intra-network and inter-network disruptions result in broad imbalances and cognitive dysfunction.
From the intra-network perspective, Zhou et al. (2012) have shown that mTBI leads to altered connectivity within the Default Mode Network (DMN), marked by reduced connectivity in posterior regions like the posterior cingulate cortex (PCC) and increased connectivity in the medial prefrontal cortex (mPFC). This imbalance between anterior and posterior regions of the DMN was closely linked to deficits in executive function and mental flexibility, suggesting that such network disruptions may be at the root of some cognitive difficulties commonly observed in mTBI patients. The hyperconnectivity within the mPFC demonstrated an inverse relationship with mood related symptoms such as depression and anxiety. It was further interpreted that the mPFC may initially compensate to sustain cognitive abilities; however, over time, this could contribute to the emergence of psychological symptoms like anxiety and depression.
From an inter-network perspective, Liu et al. (2024) note that it is typical for there to be increased dysconnectivity between the DMN and CEN in mTBI, which has been correlated to reduced working memory performance; abnormal coupling between the CEN and SN which has related to increased emotional dysregulation and internetwork irregularities between the SN and DMN, which has led to disinhibition. Their novel insights come from analyzing the neurodynamic imbalances from a temporal perspective. In comparison to healthy controls, the mTBI group spent the most time in a state characterized by reduced connectivity between the DMN and SN (state 1), whose length of time correlated to reduced scores on the Montreal Cognitive Assessment. Significantly less time was found in a state characterized by higher DMN connectivity, and negative correlation between the DMN and SN (state 3) whom the authors hypothesized may relate to reduced social cognitive abilities. Finally, mTBI participants also demonstrated overall reductions in the amount of transitions between networks.
Disruptions in other networks such as the SN and CEN have also been observed in mTBI. Liu et al. (2020) documented that mTBI patients frequently exhibit hyper-connectivity between the DMN and SN, potentially acting as a compensatory strategy to preserve cognitive performance in the wake of injury. However, this hyper-connectivity may eventually become maladaptive, contributing to further network imbalances and cognitive decline over time. The impact of these network disruptions is not confined to isolated cognitive functions. Research by Li et al. (2020), Li F. et al. (2023), Li X. et al. (2023), and Li C. et al. (2023) demonstrates that mTBI can disrupt connectivity across multiple networks, including the DMN, SN, CEN, and SMN. These disruptions are strongly associated with impairments in attention, executive function, and memory, highlighting the role of network integrity in the cognitive recovery process following injury. Specifically, decreased connectivity between the SN and executive control regions, such as the superior frontal gyrus, relate to the challenges mTBI patients face in maintaining cognitive performance.
In parallel, Rigon et al. (2016) found that mTBI leads to significant reductions in inter-hemispheric functional connectivity (FC) within externally oriented networks (EONs) such as the fronto-parietal network (FPN) and executive control network (ECN), rather than within the DMN or sensorimotor network (SMN). These specific disruptions in inter hemispheric connectivity were associated with impairments in visuospatial and organizational skills, as evidenced by poorer performance on the Rey-Osterrieth Complex Figure Test (ROCFT), implicating inter-hemispheric FC within executive control and flexibility (Rigon et al., 2016).
Structural damage, such as diffuse axonal injury (DAI), significantly impairs key neural networks like the DMN, SN, and CEN. DAI, which occurs in about half of all severe head trauma cases, involves extensive white matter damage that is correlated with cognitive deficits (Aquino et al., 2014). The severity of DAI correlates with its location: mild cases typically involve the frontal and temporal lobar white matter, moderate cases affect the corpus callosum, and severe cases extend to the dorsolateral midbrain. This damage to white matter tracts, particularly in regions like the corpus callosum and midbrain, is a primary contributor to the network disruptions observed in mTBI, leading to cognitive impairments (Aquino et al., 2014).
While the previous studies focused on spatial dynamics between and within networks of the brain, Alhourani et al. (2016) explored temporal dynamics, focusing on frequency-specific changes in connectivity following mTBI. Their study found that mTBI reduces alpha band connectivity and generates slow delta waves, both associated with white matter deafferentation and subsequent cognitive impairments. These frequency-specific disruptions, particularly within the DMN, are linked to deficits in higher cognitive functions such as memory and attention, which are commonly reported post-concussion (Alhourani et al., 2016). Additionally, the observed network topology changes, including the loss of inter-hemispheric connections, may be related to DAI's impact on white matter tracts like the corpus callosum, further exacerbating cognitive deficits in mTBI patients (Alhourani et al., 2016).
Neuropsychological functions and TBIsIn the context of such foundations as what TBIs are, how they are classified, and how the brain is typically impacted from multiple perspectives, specific neuropsychological functions may now be reviewed in depth. Damage to specific areas (i.e., frontal-temporal cortices and hippocampus) produce the deficits associated with sustaining TBI. As the previously mentioned areas are most vulnerable, the following impaired functions discussed are hallmarks of TBI.
Wang et al. (2021) found support through their research that information processing, memory, and attention are impaired. Individuals with TBI show hyperactivation in the prefrontal cortex, which can lead to cognitive fatigue compared with healthy controls (Gillis and Hampstead, 2015). A review by Blennow et al. (2016) found that impairment of the prefrontal cortex presents difficulty concentrating and poor memory. Additional symptoms related to all severity levels of TBI include nausea, dizziness, vomiting, sensitivity to light, and headaches. Mood changes can also be seen in TBI patients such as an increase in irritability. The treatment of cortical and structural areas resulting in improved functioning that had been damaged by TBI reinforces the role the brain regions play in a healthy brain and what processes are disrupted upon injury.
The prefrontal cortex connects to the limbic system and facilitates top-down processing; damage of these connections correlates to deficits in emotional processing (van der Horn et al., 2015). Emotional impairments are common symptoms of TBI and deficits in processing positively correlate with impairments in accurately identifying negative emotions in individuals with TBI (Rosenberg et al., 2015). Individuals may experience decreased emotional responses or inability to control stronger emotions such as anger (Rassovsky et al., 2015). Axonal shearing of white matter tracts is related to a decrease in general processing speed (Boccia et al., 2022; Ferraracci et al., 2021). This disruption may correlate to damage to the prefrontal cortex. Individuals with TBI compared with healthy controls have demonstrated slower processing speeds (Dymowski et al., 2015). In addition, slowed processing speed, induced by TBI, can affect working memory (Gorman et al., 2016).
TBI may also lead to affective disorders, with anxiety, depression, and PTSD being the most common. Anxiety symptoms, irritability, fatigue, and cognitive deficits persist well-beyond 3 months after the initial impact of TBI (Lamontagne et al., 2022; McMahon et al., 2014). Deficits in language and verbal memory occur consistently with TBI (Wang et al., 2021; Ryan et al., 2015b). The duration of these deficits may depend on the age of the individual when they sustained a TBI and the severity classification. Adults aged 18–24 with TBI had language processing return to normal functioning by 6 months post-injury (Coffey et al., 2021). However, other studies suggest that patients/individuals aged 5–15 years may suffer from language deficits for up to 2 years after the initial impact of TBI (Ryan et al., 2015b). The severity of TBI correlates to the severity of symptoms experienced. Age and severity are variables affecting the degree of potential recovery from damage caused to the brain. Continued research in this area could be useful in determining rehabilitation techniques to reduce the duration of deficits after TBI.
Classifying the severity of TBIsThe severity of a TBI is a significant factor that affects both the outcomes and therapies for patients (Rauchman et al., 2022). The degree of severity may increase the risk for cognitive deficits, motor impairment, and emotional difficulties (depression, anxiety, aggression, impulse control, etc.), both temporarily and permanently (Mckee and Daneshvar, 2015). Understanding the similarities and differences between these severity levels is crucial for accurate diagnosis, prognosis, and potential treatment methods. Table 1 is a table which his typically used to help classify the severity of a brain injury. Neuropsychologists are responsible for determining the length of Post-Traumatic Amnesia (PTA), a state of discontinuous cognitive functioning, classically characterized by anterograde and retrograde amnesia. One common task administered is the Galveston Orientation and Amnesia Test (GOAT), whereby the TBI patient must receive a score of 75 or greater on three consecutive trials on independent days to be determined out of PTA.
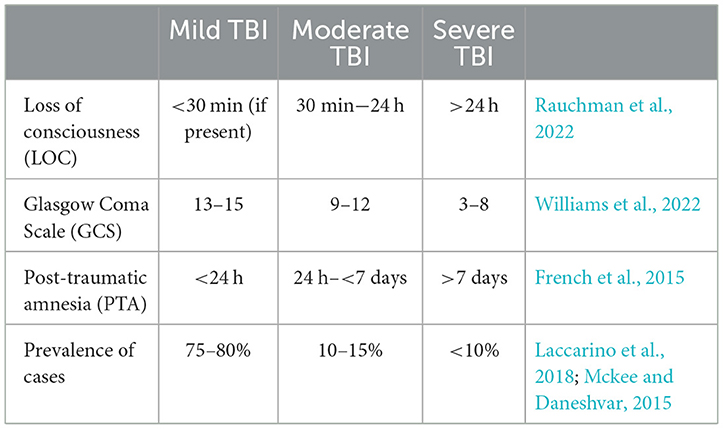
Table 1. TBI severity, classifications, and characteristics.
Imaging tools play a crucial role in determining the severity of TBI and guiding appropriate treatment strategies. These tools provide valuable insights into the structural and functional changes that occur in the brain following an injury (Mckee and Daneshvar, 2015). By visualizing the affected areas and their extent, imaging techniques enable healthcare professionals to accurately assess the severity of TBI, identify potential complications, and monitor the progression of the condition over time. The following table (Table 2) provides a summary of commonly used techniques and their advantages.
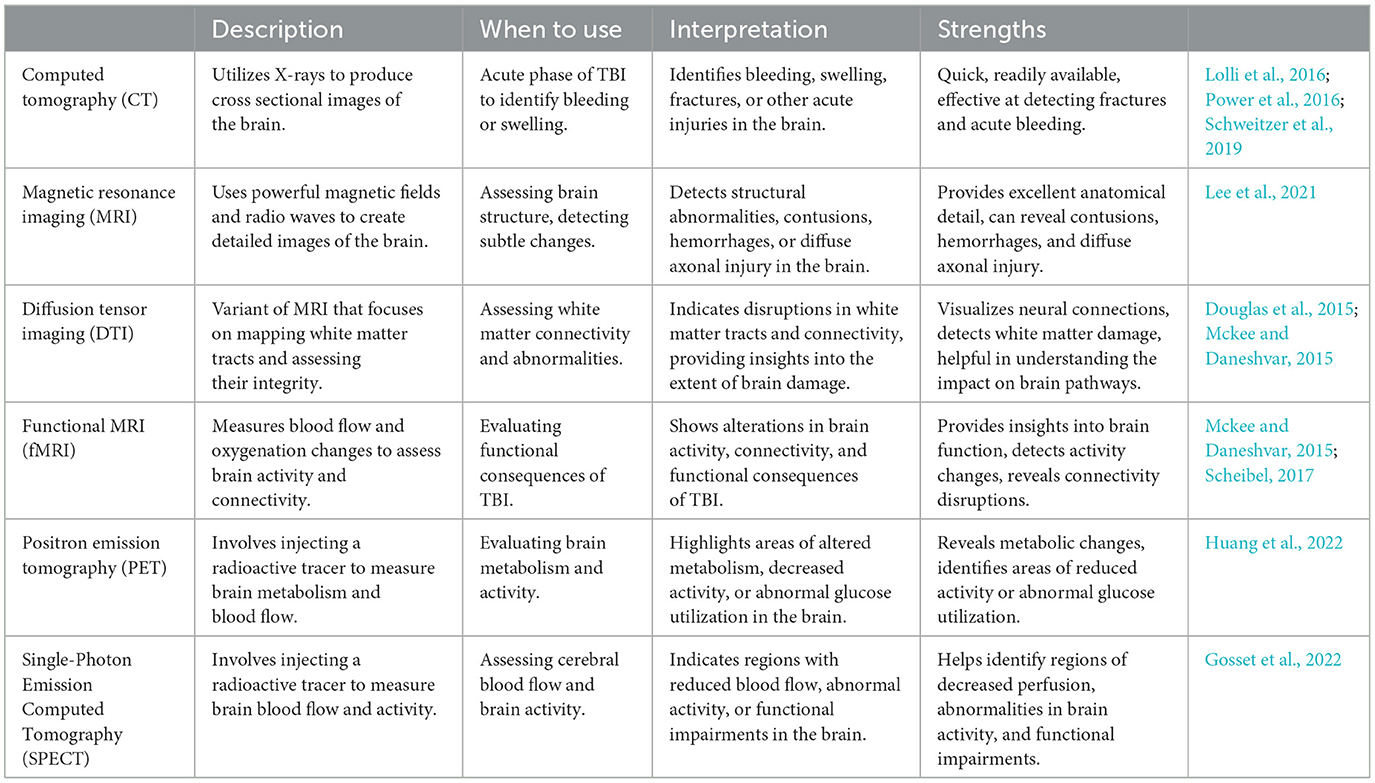
Table 2. TBI imaging tools.
Severity of TBIs and neuropsychological profileThe complexity of TBI-related impairments encompasses cognitive deficits, functional limitations, and behavioral changes. Cognitive deficits, including attention, memory, and executive function impairments, pose challenges in cognitive processes. Functional limitations affect individuals' ability to perform daily activities independently, while behavioral changes can have implications for emotional wellbeing and social interactions (Devi et al., 2020). Behavioral problems following TBI present a significant challenge, yet interventions targeting these problems have received limited attention compared with cognitive and functional deficits (Yeates et al., 2017). Treatment approaches primarily focus on addressing the cognitive and functional aspects of TBI, which can have a profound impact on an individual's daily functioning, work performance, and overall quality of life. Various injury-related factors, such as TBI severity, complications, pre-existing injuries to other body regions, and the duration of the injury, influence the manifestation of TBI symptoms (Rabinowitz and Levin, 2014).
Traumatic brain injury ranges from mild, moderate to severe. Mild TBI (mTBI) is more commonly referred to as concussions. Symptoms experienced in mTBI typically do not exceed 3 months, but they tend to subside within 7–10 days. In cases of mild TBI, individuals may experience temporary cognitive impairments, including difficulties with attention, memory, and information processing speed. Additionally, they may encounter mild functional limitations, such as changes in coordination, balance, and fine motor skills. Fortunately, these deficits are usually transient and tend to resolve relatively quickly. Understanding the neurobiological underpinnings of cognitive impairments and emotional changes following TBI provides valuable insights into the mechanisms involved. Research suggests that mTBI can lead to alterations in synaptic function and plasticity. Disruptions in synaptic strength, which refers to the ability of neurons to communicate effectively, can impair neural communication and impact cognitive processes and overall brain function (Witowski et al., 2019). Rapid changes in synaptic strength may be a contributing factor to attention deficits, memory problems, and learning difficulties commonly observed following mTBI.
In moderate TBI cases, cognitive impairments tend to be more pronounced and long lasting, involving attention and memory difficulties, executive function deficits, and reduced information processing speed. Functional impairments in moderate cases may include persistent motor coordination difficulties, challenges in performing activities of daily living, and emotional and behavioral changes. Severe TBI often leads to severe and persistent cognitive impairments affecting multiple domains, such as attention, memory, language, problem-solving, and executive functions. Furthermore, functional impairments in severe cases can manifest as severe physical disabilities, significant limitations in self-care tasks, difficulties with speech and swallowing, and cognitive and behavioral impairments (Mckee and Daneshvar, 2015). TBI also disrupts the balance of neurotransmitters in the brain, leading to alterations in mood regulation and cognitive functioning (Ahmed et al., 2017). Severe TBI can disrupt emotional contagion, making it difficult for individuals to empathize with others' feelings and maintain social relationships (Rushby et al., 2013). This means they may struggle to pick up on non-verbal cues, misunderstand emotional expressions, and react inappropriately in social situations. This impairment is linked to damage in key brain regions responsible for emotional contagion, like the amygdala and prefrontal cortex (Rushby et al., 2013).
When assessing TBI, different approaches can be taken. As previously discussed, imaging tools are a proficient manner in which to examine this condition and see physical damage to the brain. Another approach to gauging the severity of TBI is to specifically look for difficulties with attention, memory, processing speed, working memory, coordination, and executive functioning. Pen and paper, and computer tests that are administered by a trained professional can acquire such information. These tests inform the clinician to what degree the patient may be experiencing cognitive impairments. Deficits in cognitive functioning are seen in acute and chronic phases of TBI (Tsai et al., 2021).
As research accumulates, so does the opportunity exist for the synthesis of literature that may enable a convenient path for more precise neuropsychological testing. As an example, previous meta-analytic work demonstrates that particular neuropsychological functions were strongly correlated with functional outcome. Allanson et al. (2017) found that delayed verbal memory, visuo-spatial construction, set shifting, and generativity particularly stood out as significant predictors of functional outcome. A more recent meta-analysis supports and builds upon these findings (Krynicki et al., 2023), some of which is presented in Table 3.
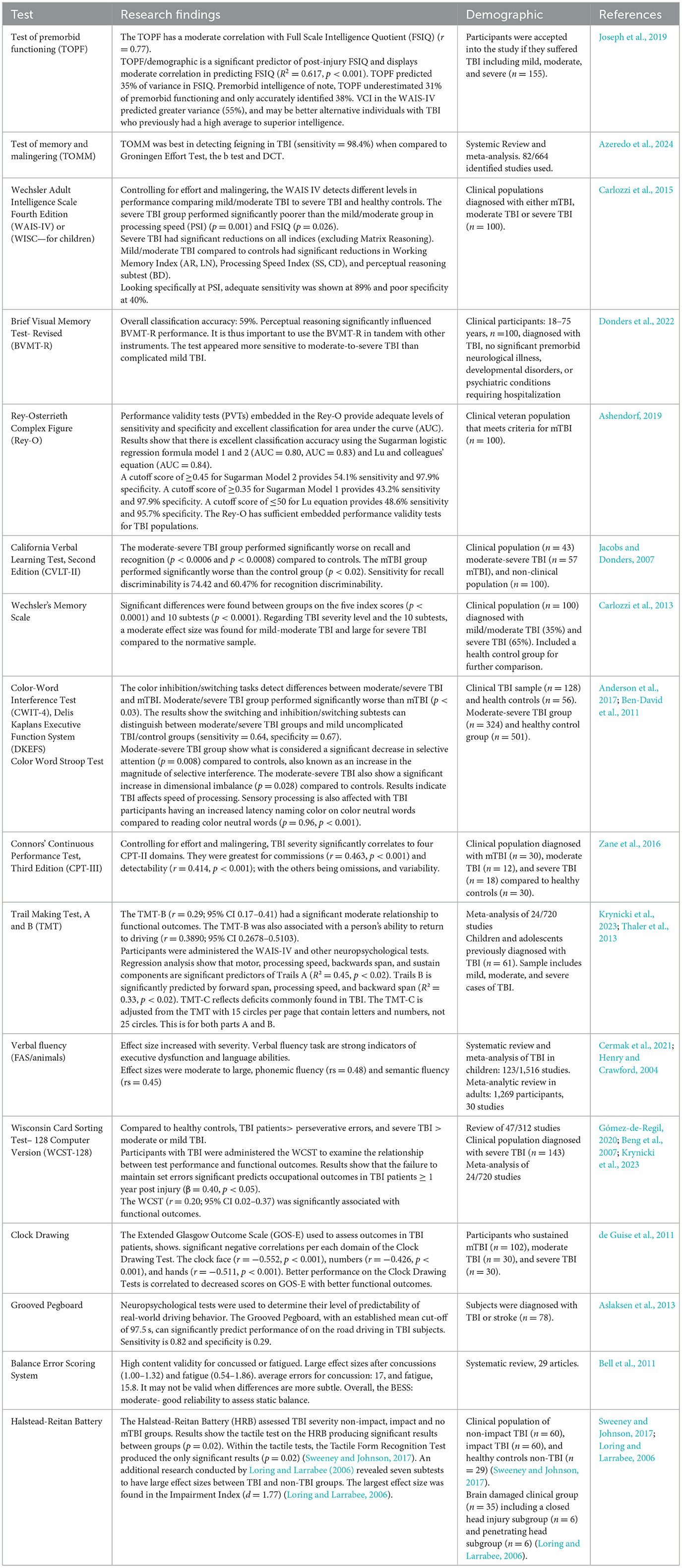
Table 3. TBI cognitive function diagnostic tools.
TBI impairs various cognitive functions including memory and executive functions. Alongside these cognitive deficits, TBI has been associated with altered affect and subjective emotional states. Forceful trauma may disrupt neural substrates and subsequently neurotransmitters altering emotions (Ahmed et al., 2017). Often, emotional disruptions experienced following TBI are displayed as behaviors and emotional reactions that cannot be accurately classified by the Diagnostic and Statistical Manual for Mental Disorders (DSM-V) (Shields et al., 2015). Decreased executive functioning coupled with the disruption of neurotransmitters as a result of TBI can have profound effects on emotional regulation. Large correlations are shown between emotional regulation and executive functioning self and informant report forms in an acquired brain injury sample of 64% were a result of TBI (Stubberud et al., 2020). Screening for emotional deficits after TBI improves the care clinicians provide. Emotional measures adequate for assessing mood disruptions in TBI populations is provided in Table 4.
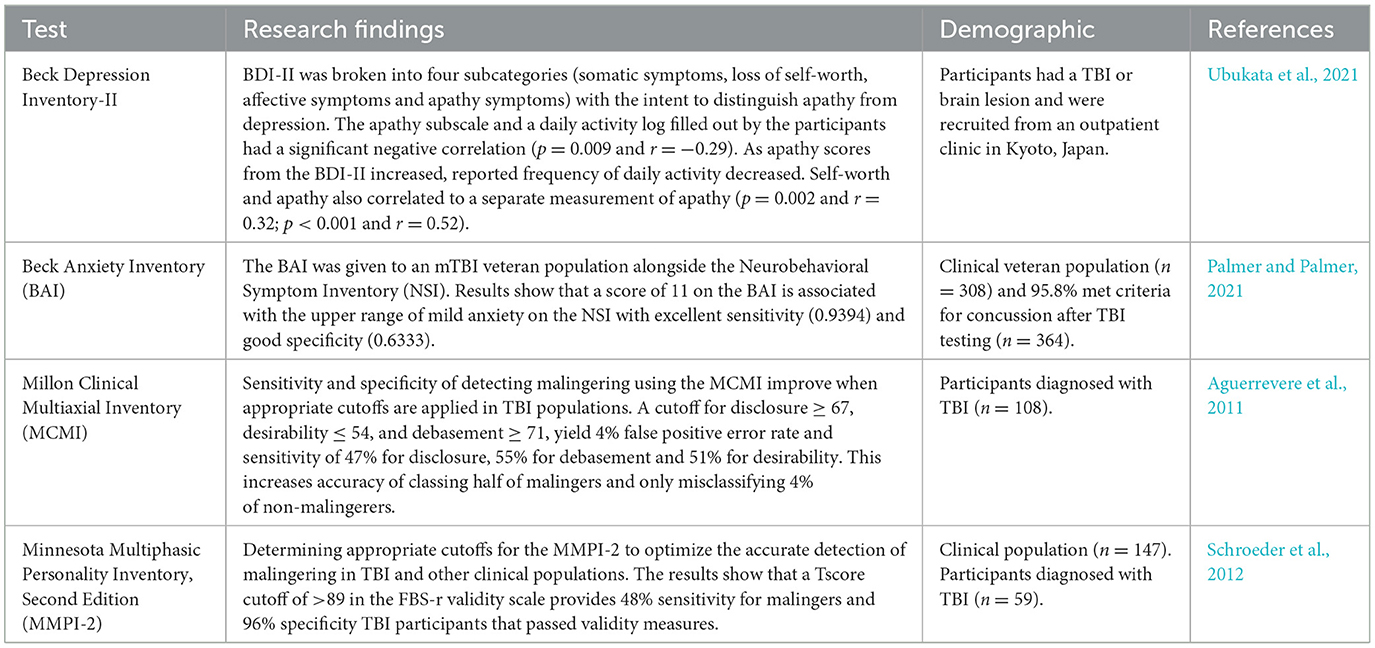
Table 4. TBI emotional function diagnostic tools.
Recovery in children, adolescents, adults, and elderlyThe trajectory of healing after traumatic brain injury (TBI) is significantly influenced by an individual's developmental stage. These stages—childhood (ages < 10), adolescence (ages 10–17), adulthood (ages 18–64), and late adulthood (ages 65+)—mark distinct phases characterized by differing resilience levels and recovery challenges post-TBI. Tailoring interventions to suit these stages optimizes rehabilitation by addressing specific developmental needs and challenges, ensuring more effective functional outcomes and enhanced quality of life.
Many studies suggest that TBI sustained in early childhood tends to have more profound and persistent effects on neuropsychological, psychosocial, and educational outcomes compared with TBI experienced in later childhood (Treble-Barna et al., 2017; Sariaslan et al., 2016; Wade et al., 2016). Younger age and greater TBI severity are linked to poorer functional outcomes (Treble-Barna et al., 2017; Sariaslan et al., 2016; Wade et al., 2016). Age of injury is a critical factor as young children have an increased vulnerability to diffuse brain injury and the harmful effects that such injury may have on their growth and development (Treble-Barna et al., 2017). It is suggested that skills that are rapidly developing during the phase of injury are more susceptible to compromise. Consequently, childhood TBI may pose an increased risk of enduring impairments. TBI severity is another critical factor in functional outcomes, as greater severity is linked to lifelong impairments (Treble-Barna et al., 2017; Sariaslan et al., 2016; Wade et al., 2016). Children with moderate to severe TBI tend to display poorer functioning in different domains, including academic performance, community engagement, interpersonal behavior, emotional state, and cognitive processing (Wade et al., 2016). Sariaslan et al. (2016) found that the risks of disability pension and psychiatric inpatient hospitalization were increased by 106 and 92%, respectively, for individuals who had experienced moderate to severe TBI during their childhood, compared with their unaffected siblings. Another study indicated that children who sustained a severe TBI during childhood displayed impairments in adaptive functioning, based on neuropsychological tests and interviews, 6.9 years post-injury (Tomar et al., 2018). These children displayed deficits in fluid reasoning and processing speed, which predicted risk of poorer outcomes in adulthood. Deficiencies in fluid reasoning may hinder an individual's ability to generate solutions and adapt their thinking to navigate daily problems. At the same time, a decrease in processing speed may interfere with daily tasks like interpreting verbal orders or dealing with the demands of a rapid work environment (Treble-Barna et al., 2017). Jones et al. (2018) found that predictors of poor cognitive function in children 12 months post-injury include low socioeconomic status, male gender, living in rural areas, and having experienced a non-accidental injury.
Within 1 month post-injury, children with mild TBI experienced minimal changes in cognitive recovery and quality-of-life (QoL) (Jones et al., 2018). However, by the 6-month mark, there was observable progress in behavioral adjustment, reflecting improvements from baseline. Similarly, 12 months post-injury, children displayed significant cognitive, behavioral, and QoL improvements.
Studies on moderate-to-severe TBI tend to be grouped together. Kennedy et al. (2022) found that older age at the time of trauma correlated with increased mortality and unfavorable overall recovery in children with moderate-to-severe TBI at 6 months post-injury. However, other studies have distinguished the differences between moderate and severe TBI. Those with severe TBI at the 6-month mark show a deterioration in the ability to copy or memorize complex visual materials, suggesting an increased susceptibility to challenging perceptual tasks (Recla et al., 2013). At 24 months post-injury, children with moderate TBI often face difficulties with short and long-term memory in both verbal and visual domains (Catroppa and Anderson, 2007). Whereas, those with severe TBI functioning worsened during the first year, as shown by their accelerated growth curves, indicating an increased presence of executive function dysfunction, specifically related to emotional control, inhibition, and working memory (Keenan et al., 2021). Severe TBI symptoms also displayed a secondary worsening at 24 months.
TBI presents with considerable heterogeneity across different individuals, as factors such as the severity of the injury, location of brain damage, age, sex, and pre-existing health conditions can all influence the manifestation and outcome of the injury, leading to highly variable outcomes (Walker et al., 2018).
Studies have found that TBIs during adolescence may be associated with more difficulties in recovery than in young children and adults because adolescence is known as a critical window for plasticity, substantial maturation, and growth (Mulligan et al., 2021). During adolescence, cortical structures undergo extensive remodeling through the process of synaptic pruning, corresponding to a decrease in cortical gray matter, acceleration of myelination of axons, increased axon density, and increased white matter volume. These changes allow the acquisition of higher cognitive functions, such as improved cognitive control, enhanced behavioral regulation, and better social cognition (Mulligan et al., 2021). The development of social skills during adolescence is critical for group membership and connection to others, which can significantly impact psychological wellbeing (Di Battista et al., 2014; Mulligan et al., 2021).
Because adolescence represents a crucial stage in brain development and maturation, any disruption to this process may have far-reaching consequences beyond the initial injury phase, leading to lasting impairment. The long-term effects of adolescent mTBI can involve attention and executive function deficits (Gutiérrez-Ruiz et al., 2022). Gutiérrez-Ruiz et al. (2022) found that adolescents with mTBI showed lower cognitive processing speeds and a decreased capacity for selective and sustained attentional tasks. These adolescents also showed an increase in symptoms related to anxiety, depression, withdrawal, and social issues (Gutiérrez-Ruiz et al., 2022). Adolescents with moderate or severe TBI experience continuous and significant declines in overall quality of life due to the impact of TBI on important domains of life, such as school, career opportunities, work functioning, and social interactions (Mulligan et al., 2021). Moreover, alterations in self-perception were observed, encompassing feelings of inferior intelligence and self-consciousness resulting in diminished social identity while increasing their reliance on others. These findings are similar to research on adult survivors of adolescent TBI, who reported “poorer school performance, greater employment difficulties, poor health-related quality of life (HRQoL), and increased risk of mental health problems” (Di Battista et al., 2014, p. 1).
With TBI recovery in adolescents, Rivara et al. (2011) found that those with mild TBI, 3 months post-injury, showed a minor decline in quality-of-life (QoL). During follow up periods, at 12 and 24 months, these patients exhibited lower QoL scores, reaching statistical significance without reaching clinically significant levels. In another study, Ryan et al. (2015a) found that adolescents with mild TBI at 12 months post-injury exhibited minimal issues with social functioning, and their social abilities remained relatively stable over time. These studies suggest that sustaining mild TBI during adolescence results in more favorable recovery outcomes.
Adolescents with moderate-to-severe TBI, 3 months post-injury, showed a significant reduction in the range of activities, social and community-based, that they could engage in. Additionally, they experienced a reduction in their communication and self-care abilities compared to their initial baseline measurements (Rivara et al., 2011). At 12 months post-injury, adolescents with moderate-to-severe TBI showed improvement in the range of activities they could participate in, although significant impairments still persisted. Additionally, at the 24- month mark, there were some minor improvements; however, their Pediatric Quality of Life Inventory scores remained significantly lower when compared to baseline. Upon further observation, these patients' communication and self-care abilities showed no significant improvements (Rivara et al., 2011). Between the 12- and 24-month post-injury period, adolescents with severe TBI experienced a significant increase in social problems (Ryan et al., 2015a).
The factors associated with the healing trajectory for TBIs in adulthood include patient characteristics, such as a history of recurrent mTBIs, younger age, and greater educational attainment (List et al., 2015; Rabinowitz et al., 2018). List et al. (2015) found that adult patients with recurrent TBIs had a higher prevalence of cognitive deficits for those who had sustained three or more mTBIs compared with those who had experienced 1 to 2 mTBIs.
Recurrent mTBIs are seen as a risk factor that contributes to the development of dementia later in life. In some individuals, mTBI may even lead to an earlier onset of Alzheimer's disease (AD). The increased risk of AD may be due to accelerated neurodegeneration caused by TBI-induced neurotoxic processes, inflammatory processes, and the accumulation of hyperphosphorylated tau. However, younger adult age has been found to be correlated with more rapid improvement than older age (Rabinowitz et al., 2018). Education has also been indicated as a moderating factor in post-injury function. Rabinowitz et al. (2018) found that participants' education level had a significant effect on their processing speed (PS), executive function (EF), and verbal learning (VL) but did not have an impact on their recovery trajectory. In comparison, younger age was correlated with better recovery of both simple and complex PS and EF. This may indicate that age is a protective factor in the recovery of TBI. However, recurrent injury leads to dose-dependent cortical thinning, resulting in detrimental effects on cognitive function that can contribute to the development of AD (List et al., 2015).
With TBI recovery in adults, Othman et al. (2022) found that 19.2% of adults with mild TBI, 3 months post-injury, showed cognitive impairment, while 19.2% exhibited neuropsychiatric manifestations. At 6 months post-injury, patients continued to experience persistent cognitive impairment, while the remaining majority showed signs of recovery. In another study, Scholten et al. (2015) found that at 6 and 7 months post-injury, adults with mild TBI exhibited significantly more favorable outcomes in various domains, such as Physical Component Summary (PCS) scores, physical functioning, role physical, social functioning, and role emotional. At 12 months post-injury, patients demonstrated outcomes that were similar to population norms, indicating a substantial level of recovery.
In adults with moderate TBI, 3 months post-injury, 39.3% of patients presented cognitive impairment, while 25% exhibited neuropsychiatric manifestations (Othman et al., 2022). At 6 months post-injury, no patients exhibited persistent cognitive impairment or neuropsychiatric manifestations. However, the study by Scholten et al. (2015) found that functional outcome and Health-Related Quality of Life (HRQL) were notably lower when compared to the outcomes observed after mild TBI. Notably, when examined 12 months post-injury, patients with moderate TBI scored higher on physical functioning, general health, and vitality on the Short-Form Health Survey (SF-36; reflecting physical, mental, and social functioning) than those with mild TBI. However, Glasgow Outcome Scale Extended (GOSE) scores were significantly lower than for those with mild TBI (Scholten et al., 2015).
Wilkins et al. (2019) found that in adults with severe TBI, 3–6 months post injury, 43% of survivors demonstrate improvement in their outcome, transitioning from unfavorable to favorable. Interestingly, 6 months post-injury, severe TBI patients scored higher in nearly all SF-36 domains and reported higher PQoL scores than moderate TBI (Scholten et al., 2015). This could be attributed to survivors of severe injuries perceiving some of their challenges as less difficult or their gratitude for being alive potentially outweighing concerns about their functional abilities. However, their functional outcome and HRQL were notably lower compared to the outcomes observed after mild TBI (Scholten et al., 2015). Twelve months post-injury, patients with severe TBI had significantly lower outcomes than mild TBI in the SF-36 domains of PCS, physical functioning, role physical, social functioning, and role emotional. Additionally, GOSE was significantly lower for severe TBI than mild TBI (Scholten et al., 2015). This observation aligns with the study by Wilkins et al. (2019), which reported that 38% of patients with severe TBI improved from unfavorable to favorable outcomes from 12 to 24 months.
When examining traumatic brain injuries (TBIs) in late adulthood, it becomes apparent that the healing trajectory is worse. The majority of the literature indicates an increase in mortality rates and poor functional outcomes. Elderly adults experience worse outcomes after TBIs due to their age, which is found to be an independent risk factor (Prasad et al., 2018; Toth et al., 2021). As individuals age, the brain undergoes atrophy, which leads to an increase in the distance between the brain and skull, making the dural vessels more susceptible to shearing damage. Many elderly patients may live with medical conditions that are masked by TBIs, and their decreased cerebral reserve makes them more vulnerable to minor injuries (Prasad et al., 2018). For instance, if an individual with advanced dementia suffers a head injury, it can lead to cognitive impairments that may prevent independent living. There is a lack of clear guidelines for the treatment of elderly TBI, and the existing guidelines are primarily based on studies of younger adults. This contributes to higher mortality rates and poor functional outcomes in elderly patients. Prasad et al. (2018) further found that, compared with younger patients, elderly patients typically have more extended rehabilitation stays, higher total rehabilitation costs, and a lower rate of improvement in functional measures. Aging and TBI also increase the risk of developing cerebral microbleeds (CMBs). Toth et al. (2021) found that both aging and TBIs can cause CMBs through mechanisms such as cerebrovascular oxidative stress, matrix metalloproteinase activation, and changes in the cerebrovascular wall. CMBs impact the healing trajectory of the elderly because they may lead to “cognitive impairments, psychiatric disorders, and gait dysfunction” (p. 1).
The prognosis for recovery following traumatic brain injury in older adults is generally poor, with substantial evidence indicating high mortality rates and limited functional independence. TBI recovery in older adults shows that those with mild TBI, 3 months post injury, exhibited lower cognitive functioning and performed worse on neuropsychological tests compared to non-injured peers (Hume et al., 2023). At 6 months post-injury, 14% of older adult patients with mild TBI died (Utomo et al., 2009). Thompson et al. (2020) compared older and younger adults with mild TBI, finding that from 1 week to 1 year post-injury, older adults consistently reported poorer overall physical Health-Related Quality of Life (HRQOL) than their younger counterparts.
Older adults with moderate TBI, 3 months post-injury, experience significantly poorer functional health status, with an average Glasgow Outcome Scale Extended (GOSE) score of 5.1, indicating a greater degree of disability following a moderate TBI compared to younger adults (Thompson et al., 2020). At 6 months post-injury, 47.8% of patients with moderate TBI died (Utomo et al., 2009). One-year post-injury, older adults with moderate TBI continued to report an ongoing average of 3.9 symptoms out of 17 symptoms assessed, with a higher likelihood of experiencing balance and coordination issues (Thompson et al., 2020).
Older adults with severe TBI, 6 months post-injury, with a Glasgow score of <9 exhibited unfavorable outcomes, with a significant mortality rate of 83.3% (Utomo et al., 2009). Similarly, Maiden et al. (2020) found that 6 months post-injury, 85% had died, 47% were living dependently, and 6% had recovered to functional independence. By 12 months post injury, 86.3% had died, 6.4% were living dependently, and 7.3% had recovered to functional independence. Overall, the data reveals that older adults with severe TBI face a substantial risk of death, with only a minority regaining functional independence. The following figure (Figure 1) shows children, adolescent, adult, and elderly TBI risk factors.
Phenomenology of TBIsPhenomenological studies of TBI healing progression can provide valuable insights into the subjective experiences of individuals with TBI and the factors that shape their recovery journey. By understanding the individual's perspective on their recovery, healthcare professionals can better tailor their treatment approaches and support the individual in their efforts to recover and return to their everyday lives.
Many phenomenological studies of TBI have interviewed patients during their recovery to assess their functional outcomes and wellbeing over time. Wellbeing is typically categorized as psychological, physical, and social, but there is often overlap between these categories in the literature on TBI healing progression.
Visser et al. (2021) conducted a study examining patients' wellbeing after injury, from their experiences in the emergency room to discharge and rehabilitation. Patients who were severely injured reported feeling a fear of dying while being treated in the ambulance and emergency room. For instance, one patient (female, >16 years) feared she was going to die after seeing blood spouting from her leg, thinking it was arterial bleeding (Visser et al., 2021). Patients who were sedated, unconscious, or experiencing post-traumatic amnesia during treatment often reported feelings of confusion and anxiety about the events of their injury. The realization that they had survived the injury often came to patients during hospitalization and recovery, and this realization was frequently accompanied by a fear of permanent physical limitation, replacing the earlier fear of dying. These findings highlight the initial emotional turmoil and evolving fears faced by patients after severe injuries.
Many patients reported experiencing symptoms of acute stress disorder (ASD) during hospitalization and post-traumatic stress disorder (PTSD) during rehabilitation. Stenberg et al. (2022) followed up with patients 1 and 7 years after TBI and found that wellbeing resulted from adaptation to a recovered or changed life situation. Those with moderate to severe disabilities reported poor wellbeing because adaptation was an ongoing process. However, patients reported that adaptation and wellbeing were facilitated by factors such as leading a purposeful daily life, maintaining an optimistic perspective, and employing adaptive strategies, such as applying knowledge gained from previous hardships. For instance, when asked about the ability to adapt, one patient (Female, 36 years) expressed that despite her permanent injuries, she focused on choosing to be positive and appreciating the good things in life rather than being sad about the negative things (Stenberg et al., 2022). Developing resilience and a positive outlook helped mitigate the negative emotional impact of TBIs in patients' lives.
Experiences related to physical function also contributed to patients' wellbeing. Visser et al. (2021) found that patients often reported that the time needed for recovery was much longer than they anticipated. The desire to feel autonomous rather than helpless often motivated severely injured patients to push through physical limitations during rehabilitation. Stenberg et al. (2022) found that patients with severe TBI reported suffering from life long limitations that impact their health and wellbeing. Overcoming obstacles such as the denial of one's disabilities, feelings of guilt, shame, loneliness, and isolation, and avoidant behavior were all described as necessary for adaptation and wellbeing by severely injured patients. For instance, when asked about difficulties in adapting, one patient (Male, 27 years) expressed increasing depression and frustration due to the persistent impact of his injuries on his life (Stenberg et al., 2022). These experiences reveal the persistent challenges and emotional struggles that accompany physical recovery.
Social support is another critical aspect of TBI recovery, as it is known to improve mental health and overall wellbeing. Visser et al. (2021) found that while it was challenging to rely on others, patients were thankful for the assistance they received from loved ones. In addition, patients believed that their friends' and family's support could aid their recovery. Stenberg et al. (2022) found that living with a severe disability impacted patients' social wellbeing. Patients coping with a severe disability described it as frustrating and leading to loneliness in daily life, as it resulted in exclusion from work and social networks, and the inability to participate in leisure activities. For instance, when asked about living with a disability, one patient (Male, 57 years) described feeling extremely lonely and frustrated by his inability to communicate and participate in activities, ultimately accepting his situation with difficulty (Stenberg et al., 2022). Social support is crucial in mitigating the isolation and emotional distress experienced by TBI patients.
Defense or deficit?Phenomenological studies help “humanize” TBI accounts, especially in such reviews as this. The difficulties mentioned in previous examples, in combination with cognitive deficits summarized throughout lead to another important consideration for all neuropsychologists, especially when involved in therapeutic intervention (directly or indirectly). This is the development of the knowledge and skills necessary to help determine whether the patient's presentation is a psychological defense or deficit. These defenses can be a false positive, masking a cognitive deficit, or the cognitive deficit may mimic a defense. The following are some examples where defenses and deficits may be confused:
1. Anosagnosia, or a lack of insight into ones own impairments, may be confused with denial.
2. Executive dysfunction may lead to misattribution of blame, disinhibition and lack of self insight which could be confused with projection, acting out or displacement.
3. Rationalization may also stem from impaired judgment due to frontal compromise, as opposed to a personality type, or emotional reaction.
4. Repression or lack of
Comments (0)