Coronavirus disease 2019 (Covid-19) is a multisystem disease caused by severe acute respiratory syndrome coronavirus 2. Since the emergence of the Covid-19 pandemic, the prevention of this rapidly spreading infection became a priority for all countries. A number of different vaccines were developed based on various mechanisms: RNA vaccines (Pfizer, Moderna), viral vector vaccines (Oxford-AstraZeneca, Sputnik V, Johnson and Johnson), inactivated virus vaccine (Covaxin by Bharat Biotech) and protein-based vaccines (Novavax). Two vaccines were used in India—Covaxin by Bharat Biotech, which is an inactivated virus vaccine and the viral vector vaccine by Oxford-AstraZeneca, which was manufactured locally by Serum Institute of India as Covishield. As this was one of the largest vaccine drives with vaccines getting approved in a short time, vaccine safety was closely assessed. Covid-19 is associated with a wide variety of cutaneous manifestations including urticarial rash, chickenpox-like vesicles, erythematous maculopapular rash, pernio-like lesions, acral erythema, dactylitis, purpuric maculopapules and acro-ischaemic lesions.1 Limited literature is available on skin manifestations following the Covid-19 vaccine, especially a viral vector vaccine. We report an immunocompetent patient with herpes zoster after the first dose of the viral vector vaccine.
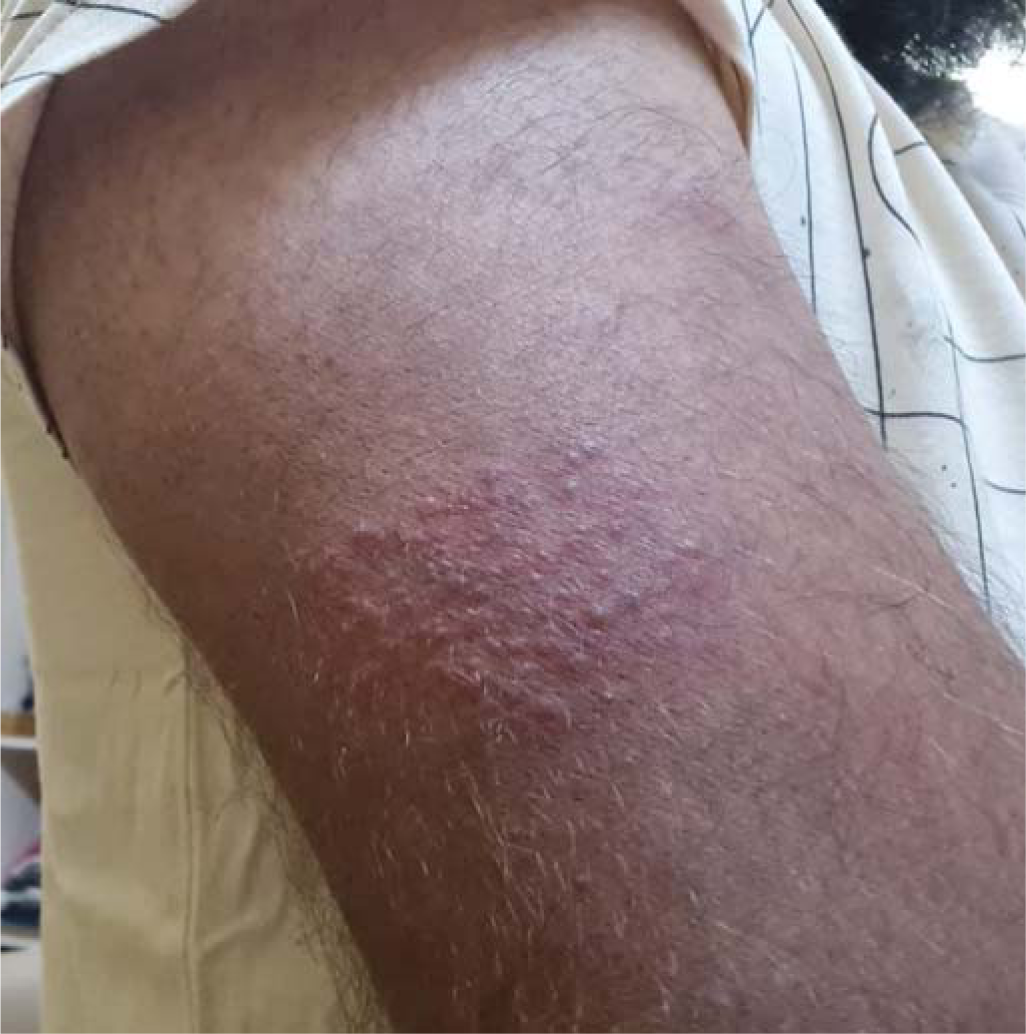
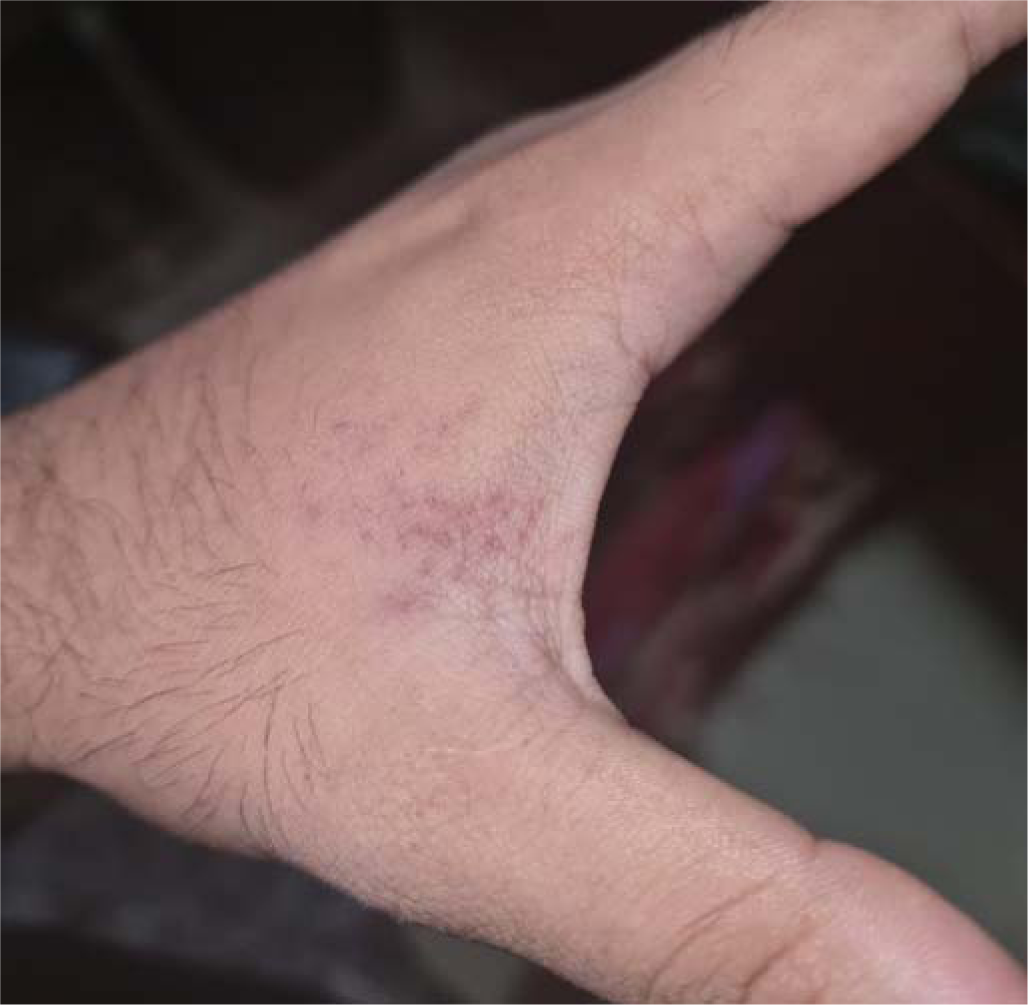
THE CASEA 24-year-old healthy man with no comorbid conditions started developing pain over the arm and forearm of the same side, extending to the thumb and index finger, 2 weeks after the first dose of inactivated virus vaccine. Two days later, he noticed small fluid-filled lesions over the arms and forearms in groups and red-coloured rash on the palms (Figs 1 and 2). Examination revealed lesions involving the C6–C7 dermatome. Our final diagnosis was herpes zoster considering the characteristic grouped vesicles associated with pain and dermatomal distribution. He was started on oral valacyclovir thrice daily for 5 days and the lesions subsided over few days with no sequelae or post-herpetic neuralgia. The second dose was taken 4 weeks after the first and the post-vaccine days were uneventful.
Export to PPT
Export to PPT
DISCUSSIONAfter a primary infection of chickenpox, the varicella zoster virus remains latent in the dorsal root ganglia or cranial nerve ganglia, which gets reactivated spontaneously or gets triggered by trauma, stress, fever or immunosuppression. Herpes zoster reactivation has been recorded post-vaccination such as inactivated influenza, hepatitis A and rabies vaccines.2 Even after Covid-19, many cases of herpes zoster reactivation were reported.3 This is thought to be due to Covid-19-induced lymphopenia and functional impairment of CD4+ T cells and activation with subsequent exhaustion of CD8+ T cells.4 Clinicians investigated patients for Covid-19 when they presented with herpes zoster, especially in those without any obvious triggers. After the vaccination drive started, there were many reports of herpes zoster getting triggered by vaccines, especially with mRNA vaccines5 and inactivated viral vaccines.6 mRNA vaccine is considered to act by stimulation of innate immunity through toll-like receptors (TLRs)3 causing reactivation of herpes zoster. The time of reactivation of herpes zoster post-vaccine varied in different reports—2 weeks in our patients, 5 days in a patient reported by Bostan et al. for inactivated virus vaccine,6 and 2 days after the first dose in case of mRNA vaccine.5 Even though it is not possible to establish a direct relationship between herpes zoster and the Covid-19 vaccine, it alerts clinicians to look for a relationship between vaccination and herpes zoster reactivation. Thus, careful documentation and reporting of cutaneous manifestations associated with the Covid-19 vaccine is important.
Comments (0)