Major depressive disorder and schizophrenia are among the top ten mental disorders globally and have a significant impact on individuals’ mental and physical well-being (1, 2). By 2015, the global prevalence of depression had surpassed 300 million individuals (3). Concurrently, research has shown a 49.86% increase in the global incidence rate of depression from 1990 to 2017 (4). Schizophrenia is widely recognized as the most severe mental disorder, as it poses a significant risk to the well-being of individuals with schizophrenia and may also endanger the safety of others (2). According to available data from reputable institutions on schizophrenia, the lifetime risk of developing schizophrenia is estimated to be 1% for the general population, and both men and women are equally affected (5). Individuals with schizophrenia frequently manifest prominent psychiatric symptoms, including delusions, hallucinations, and disordered thinking, which adversely impact their overall quality of life (6). Numerous risk factors are recognized to be closely associated with major depressive disorder and schizophrenia, but due to their complex aetiology, additional research is warranted (1, 2). Recently, many studies have shown a connection between smoking-related behaviors and severe depression and schizophrenia, which significantly contribute to the onset and progression of major depressive disorder and schizophrenia (7).
Lung cancer is a prevalent malignancy and is responsible for the greatest number of cancer-related deaths globally, accounting for approximately 2 million newly diagnosed cases and 1.76 million deaths annually (8). In 2018, the United States reported over 230,000 incident cases of lung cancer, surpassing the combined mortality of breast, prostate, and colon cancer (9). Age is a significant risk factor for lung cancer, with a greater incidence observed in males than in females (10). Furthermore, chronic lung diseases, genetic factors, air pollution, kitchen fumes, long-term smoking, and second-hand smoke are all risk factors for lung cancer, among which long-term smoking is considered an independent and important risk factor for lung cancer (11–13). Several studies have indicated a greater prevalence of smoking and increased vulnerability to nicotine addiction among individuals with severe depression and schizophrenia, which may contribute to a heightened risk of developing lung cancer. Concurrently, studies conducted by Benchalak Maneeton et al. revealed a heightened prevalence of major depressive disorder in individuals suffering from lung cancer, suggesting a potential correlation between major depressive disorder and lung cancer (14). Furthermore, research by Merete Nordentoft et al. has shown that patients with schizophrenia face an increased risk of mortality from various cancers, notably lung cancer (15). However, substantial evidence supporting the association between major depressive disorder, schizophrenia, and the risk of lung cancer remains limited (16–18). These findings accentuate the urgent need to delve into the causal connections between major depression, schizophrenia, and the elevated risk of lung cancer development.
In recent years, with the rapid development of technology, an increasing number of Genome-Wide Association Study (GWAS) databases have been utilized for scientific research (19). Moreover, Mendelian randomization (MR), a causal inference method, has wider application prospects (20). Owing to the principle of allele distribution in genetic variation, MR analysis can effectively mitigate potential confounding factors, and is often referred to as a “natural randomized controlled trial” (21). Single nucleotide polymorphisms (SNPs) strongly correlated with exposure factors can be employed as instrumental variables (IVs) to elucidate the potential causal relationship between exposure and outcomes (22). Additionally, two-sample bidirectional and multivariable MR analyses, which can serve as effective supplements to MR research, are wildly utilized to uncover certain genetic associations between exposures and outcomes (23).
To our knowledge, there is limited research using MR to investigate the causal effects of major depressive disorder and schizophrenia on the risk of lung cancer (overall, adenocarcinoma, and squamous cell). Consequently, our aim was to discover these causal relationships by utilizing two-sample bidirectional and multivariable MR analyses. Furthermore, we investigated the mediating effects of three smoking-related behaviors (smoking initiation, pack years of smoking, and cigarettes smoked per day) on the relationship between major depressive disorder and schizophrenia and the risk of lung cancer (overall, adenocarcinoma, and squamous cell).
Materials and methodsMendelian randomization study designThe Mendelian randomization study was conducted according to the STROBE-MR statement (24). Furthermore, two-sample bidirectional/multivariable Mendelian randomization and mediation analysis were employed in this study. To ensure the reliability of the study, three assumptions were made: (i) the selected IVs are strongly associated with major depressive disorder and schizophrenia, (ii) the IVs are not connected with other confounding factors, and (iii) the IVs only affect the outcome through the exposure (Figure 1) (23). Causal relationships were evaluated using the IVW method, while additional methods such as weighted median, MR-Egger, and MR-PRESSO were employed to ensure the reliability of the findings. Additionally, bidirectional MR analysis revealed a causal relationship between exposures and outcomes. Since smoking-related behaviors are important risk factors for lung cancer, multivariate MR analysis included these confounding factors. Mediation analysis was further conducted to examine the mediating role of smoking-related behaviors between exposures and outcomes. The flowchart of the study is shown in Figure 2.

Figure 1 Directed acyclic graph of the Mendelian randomization study.
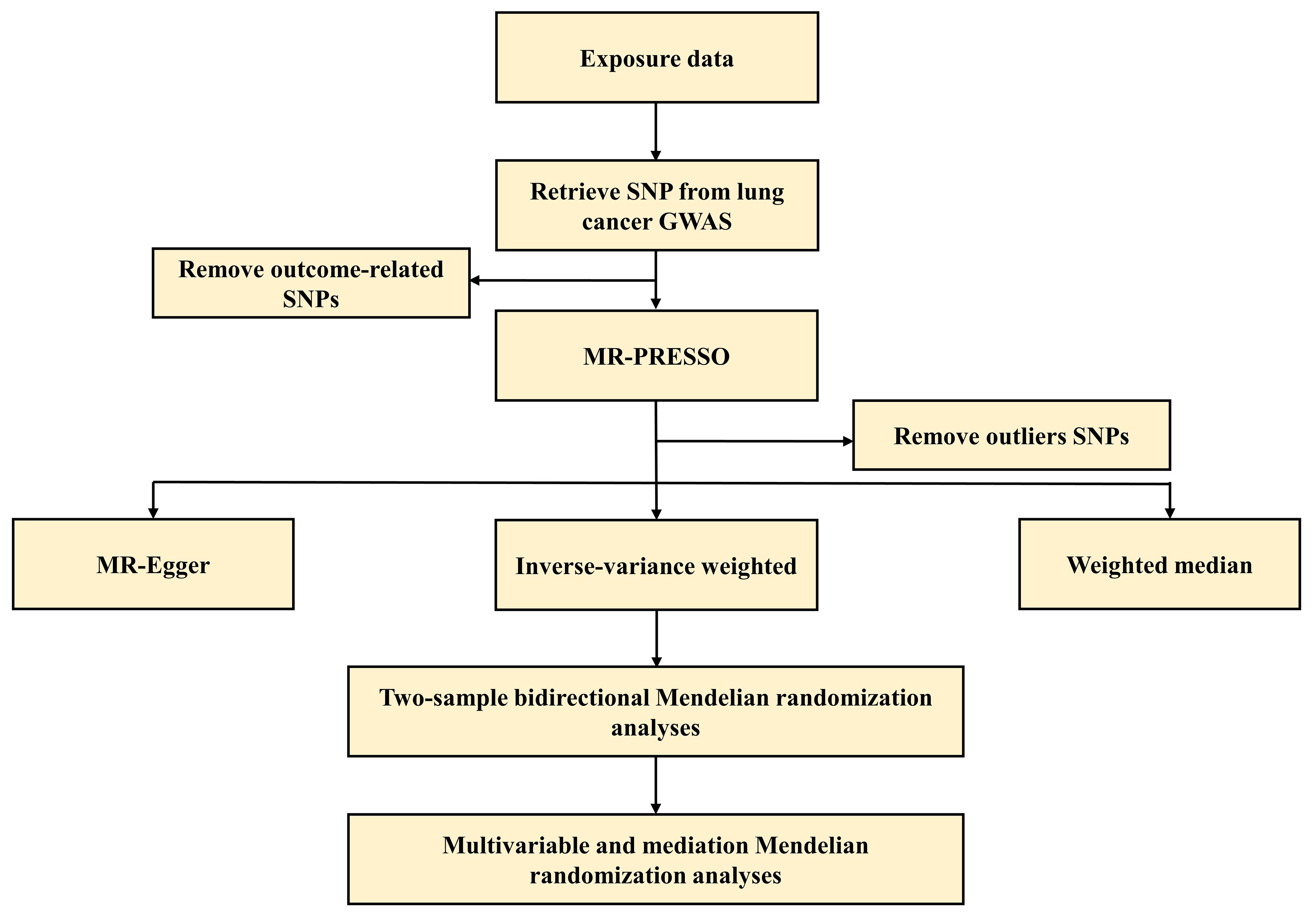
Figure 2 The flowchart of this Mendelian randomization study. GWAS, genome-wide association studies; MR-PRESSO, MR Pleiotropy RESidual Sum and Outlier; SNP, single nucleotide polymorphisms.
Exposure and outcome data sourcesData on major depressive disorder and schizophrenia were collected from two large European ancestry cohorts. This study utilized GWAS summary data from the PGC, consisting of 170,756 cases and 329,443 controls of European ancestry, to examine major depressive disorder (25). Severe depression patients in the study were defined as those diagnosed with severe depression during hospitalization or based on medical health records, or those who self-reported depression or had received relevant treatment (25). The GWAS summary data on schizophrenia were derived from the PGC consortium, which included 52,017 cases and 75,889 controls of European ancestry (26). Clinical experts diagnosed most of the patients, including individuals with schizophrenia and schizoaffective disorder, while others were diagnosed based on research-based assessments (26). Smoking-related behaviors, including smoking initiation, pack years of smoking, and cigarettes smoked per day, were considered as mediating factors. Recent WAS of smoking initiation and cigarettes smoked per day from the GSCAN consortium identified 607,291 and 249,752 individuals of European ancestry, respectively (27). In addition, a cohort of 142,387 individuals of European ancestry was used to summarize the number of pack years of smoking. These smoking-related behaviors were assessed through self-report questionnaires and initial assessments (27). Additionally, the outcome data on overall lung cancer, lung adenocarcinoma, and squamous cell lung cancer were sourced from a European ancestry cohort in the International Lung Cancer Consortium (ILCCO), which included 11,348, 3,442, and 3,275 patients, respectively (28). The outcome data were collected from a non-overlapping population to effectively minimize bias (28). Detailed information on all exposure and outcome data is provided in Supplementary Table S1.
Furthermore, recognizing the impact of the sample overlap rate between the exposure and outcome databases on the study’s results, we meticulously quantified the relevant sample overlap rate. Simultaneously, utilizing the correct methodologies (https://sb452.shinyapps.io/overlap/), we assessed the statistical bias and the likelihood of type I error resulting from the sample overlap rate (29). The results revealed that the sample overlap rate in our study was less than 10% (Supplementary Table S2), imposing a negligible influence on the research outcomes (30).
Selected IVsThe IVs were carefully selected to ensure a strong connection between major depressive disorder and schizophrenia. IVs with a P-value < 5×10-8 were considered significant and selected. Moreover, IVs with a low likelihood of linkage disequilibrium (LD) (R2 < 0.001) and a substantial physical distance (≥10,000 KB) were selected (31). A higher F-value (F > 10) was used to mitigate potential biases caused by weak instruments in this study (32). Furthermore, IVs selected for participation in this study were scanned utilizing the PhenoScanner tool (http://www.phenoscanner.medschl.cam.ac.uk/) to exclude the potential confounding factors (33); if any SNP was connected with the outcomes and confounding factors, they were removed (Supplementary Table S3). Supplementary Table S4 presents a comprehensive list of all the instrumental variables utilized in this study.
Statistical analysisAll analyses were performed using R software (Version 4.2.1). The analyses utilized the “TwoSampleMR” (Version 0.5.6), and “MR-PRESSO” (Version 1.0) R packages. All statistical analyses were conducted using a two-sided approach. To mitigate bias, a Bonferroni-corrected significance level of P-value < 0.0083 (0.05/6) was used (34). P-values between 0.0083 and 0.05 were considered suggestive of a potential association. P-values greater than 0.05 indicated the absence of a statistically significant association between the exposures and outcomes.
ResultsTwo-sample bidirectional MR studyThe IVW results for major depressive disorder and schizophrenia’s impact on the overall risk of lung cancer, lung adenocarcinoma, and squamous cell lung cancer are presented in Table 1, Figure 3. Schizophrenia was found to have a genetic connection with an increased risk of overall lung cancer (OR = 1.144, 95% CI: 1.048-1.248, P = 0.003). However, no significant associations were observed between schizophrenia and the risk of lung adenocarcinoma or squamous cell lung cancer. Similarly, there was no noteworthy association between major depressive disorder and the risk of lung cancer, lung adenocarcinoma, or squamous cell lung cancer. To mitigate potential reverse causal effects, we conducted a reverse MR analysis to evaluate the impact of the overall risk of lung cancer, lung adenocarcinoma, and squamous cell lung cancer on major depressive disorder and schizophrenia. The IVW results of the reverse MR analysis indicated no reverse causal effects of the risk of overall lung cancer, lung adenocarcinoma, or squamous cell lung cancer on major depressive disorder and schizophrenia. The results of the reverse MR analysis can be found in Supplementary Table S5.
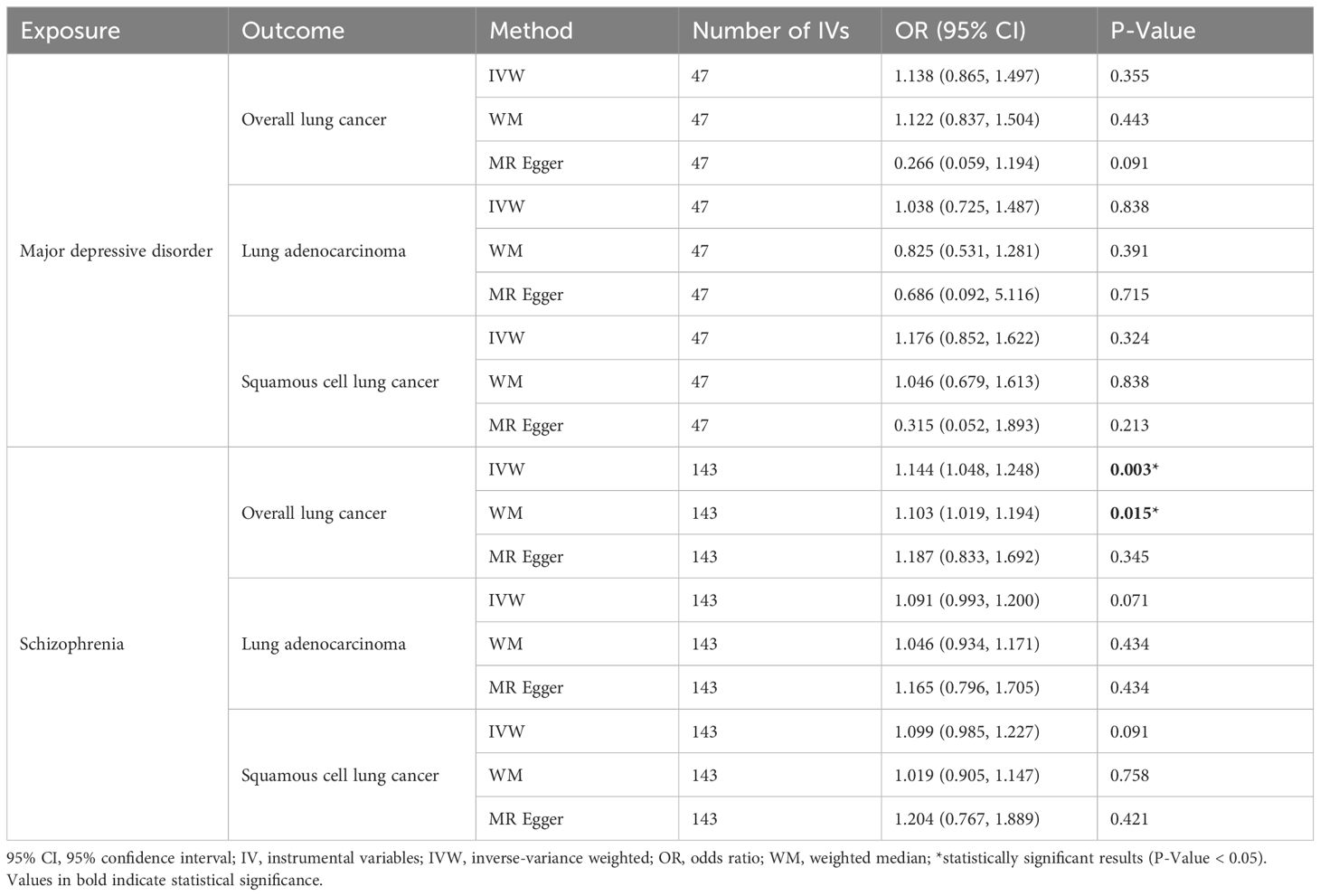
Table 1 Causal effect of major depressive disorder and schizophrenia on Lung Cancer.
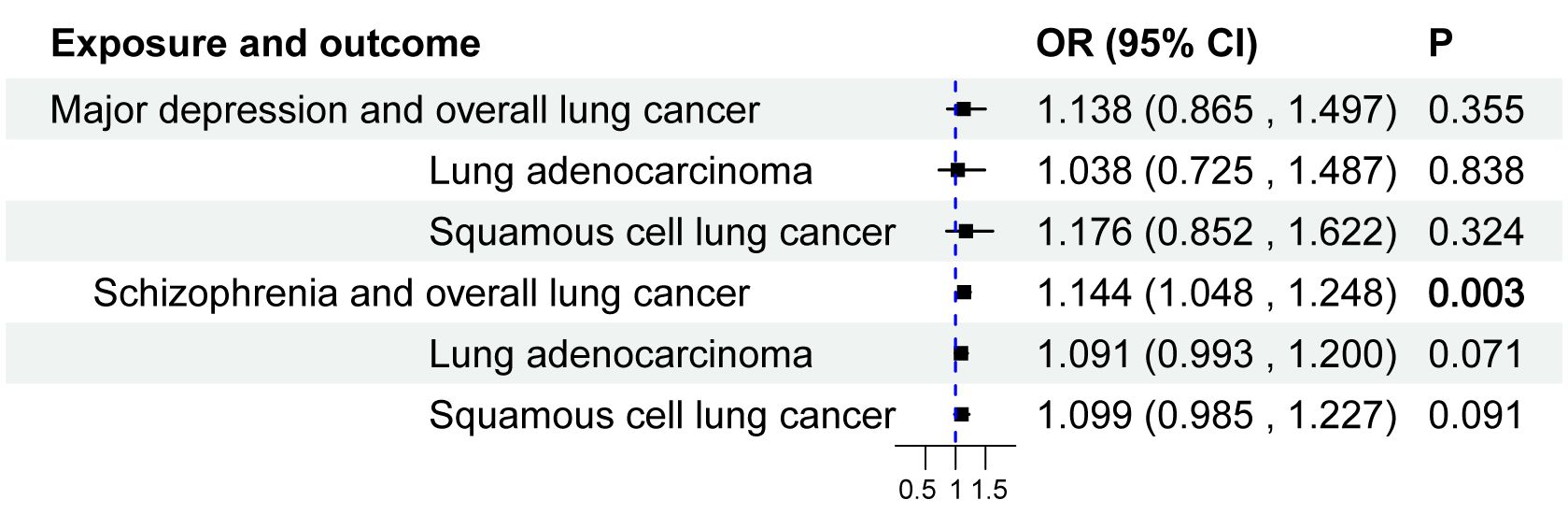
Figure 3 Causal effect of major depressive disorder and schizophrenia on the risk of Lung Cancer. 95%CI, 95% confidence interval; OR, odds ratio.
Multivariable and mediation MR studyThe multivariable MR analyses considered three smoking-related behaviors: smoking initiation, pack years of smoking, and cigarettes smoked per day. After adjusting for these smoking-related behaviors, we did not find any evidence of a causal relationship between major depressive disorder, schizophrenia, and overall risk of lung cancer, lung adenocarcinoma, or squamous cell lung cancer (Supplementary Table S6). These findings suggested that the observed effects of major depressive disorder and schizophrenia on the risk of these lung cancers are potentially mediated by smoking-related behaviors. Additionally, the mediation analysis demonstrated that cigarettes smoked per day mediated the association between schizophrenia and the overall risk of lung cancer with an OR of 1.185 (95% CI: 1.112-1.264, P = 0.021), and a mediation effect of 61.033%. The mediation effect is presented in Table 2.
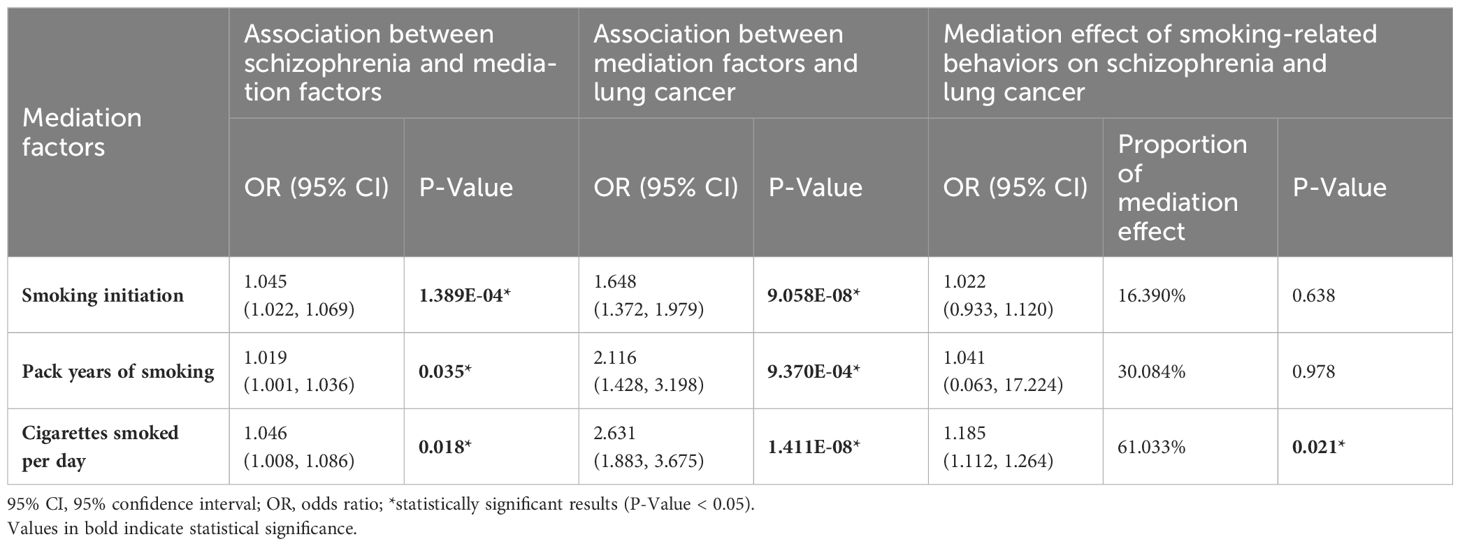
Table 2 Mediation effect of smoking-related behaviors on schizophrenia and Lung Cancer.
Sensitivity analysisThe F-values of all IVs were greater than 10, indicating their ability to mitigate potential biases (Supplementary Table S4). To assess the validity of the results obtained from this MR analysis, we conducted comprehensive sensitivity analyses, which included the heterogeneity test, pleiotropy test, and MR-PRESSO test. Theresults of these analyses revealed no horizontal pleiotropy or heterogeneity (Supplementary Tables S7, S8). Moreover, the leave-one-out plots demonstrated the causal effects of schizophrenia on the overall risk of lung cancer (Supplementary Figure S1). Additionally, both the scatter plot and funnel plot for schizophrenia and the overall risk of lung cancer indicated the reliability of the MR results (Supplementary Figures S1, S2).
DiscussionIn this two-sample bidirectional/multivariable and mediation MR study, we examined the causal relationships between major depressive disorder, schizophrenia, and the overall risk of lung cancer, lung adenocarcinoma, and squamous cell lung cancer. Based on two-sample bidirectional MR analyses, schizophrenia was found to be significantly associated with an increased overall risk of lung cancer. Moreover, we identified a partial mediation effect of cigarettes smoked per day on the association between schizophrenia and the overall risk of lung cancer, providing further insight into the impact of schizophrenia on overall lung cancer development.
Major depressive disorder and schizophrenia, two highly debilitating mental illnesses, greatly affect human health (35, 36). Numerous studies have demonstrated the detrimental effects of depression and schizophrenia on the incidence, progression, and prognosis of cancer (15, 37). Studies have suggested a potential stress pathway shared between depression and cancer, wherein certain pro-inflammatory mediators impede the regulatory feedback of the hypothalamic-pituitary-adrenal axis, which is mediated by glucocorticoids (38). Additionally, several MR studies have identified correlations between depression and the incidence and progression of prostate and breast cancer (18, 39). Nevertheless, our study did not uncover a causal link between severe depression and the risk of lung cancer (including overall, adenocarcinoma, and squamous cell types). Moreover, even after accounting for three smoking-related behaviors in the multivariate MR analysis, we observed no causal connection between depression and lung cancer risk. A 21-year longitudinal investigation revealed that major depressive disorder is linked to an increased frequency of daily smoking and higher rates of nicotine dependence; however, the causal relationship between smoking and depression remains elusive (40). Concurrently, Patrícia Pelufo Silveira et al. identified 11 gene loci in women and a single gene locus in men that were significantly associated with the major depressive disorder phenotype through gender-specific GWAS analysis of UK Biobank data. Given the notably greater incidence of lung cancer in men than in women, our inability to establish a link between major depression and lung cancer risk may stem from gender disparities. The current limitations of GWAS databases preclude further sex-specific analysis, underscoring the imperative for future sex-stratified investigations. This suggests that depression may not be significantly linked to the development of lung cancer, emphasizing the need for further research on this relationship.
A cohort study revealed a correlation between schizophrenia and the incidence of breast cancer, possibly attributed to hyperprolactinemia resulting from patients’ long-term use of antipsychotic drugs, ultimately leading to breast cancer (41). Furthermore, certain well-established antipsychotic medications, such as phenothiazines and reserpine, have exhibited anticancer effects. This finding suggests possible shared mechanisms and pathways between schizophrenia and the onset and progression of cancer (42). In a meta-analysis of 13 cohort studies with 218,076 male participants, Fan et al. reported a correlation between schizophrenia and the likelihood of developing prostate cancer (43). By utilizing data from the Swedish cohort and genome-wide association study (GWAS) data from the International Union, a study revealed a genetic association between schizophrenia and breast cancer, identifying the shared locus 19p13 (GATAD2A), and these findings indicate a genetic overlap between the two phenotypes (44). Collectively, these studies demonstrate a strong relationship between schizophrenia and the incidence and progression of cancer, emphasizing the need to examine the underlying mechanisms involved. Our two-sample bidirectional MR analysis provided robust evidence indicating an increased risk of overall lung cancer among individuals with schizophrenia. Given the greater propensity of individuals with schizophrenia to engage in smoking, which is a significant risk factor for lung cancer, we conducted additional multivariate and mediation Mendelian randomization analyses to investigate the potential impact of smoking-related behaviors on the relationship between these two conditions. After accounting for smoking behavior in the multivariate Mendelian randomization analysis, the previously observed association between schizophrenia and the risk of lung cancer lost significance, suggesting that smoking-related behavior mediated this relationship. The mediation analysis results revealed that the number of cigarettes smoked per day partially mediates the association between schizophrenia and the overall risk of developing lung cancer. These findings clarify the link between schizophrenia and the development of lung cancer while also offering novel perspectives for future mechanism-based research.
There are several strengths in this MR study. First, the GWAS summary data for major depressive disorder, schizophrenia, and the overall risk of lung cancer, lung adenocarcinoma, and squamous cell lung cancer were collected from the European population, minimizing potential biases (30). Second, the Bonferroni-corrected analysis was utilized to reduce the potential risk of type I error (34). Third, this MR study revealed for the first time a causal relationship between major depressive disorder, schizophrenia, and overall risk of lung cancer, lung adenocarcinoma, and squamous cell lung cancer.
Several limitations exist in this MR study. First, the causal connection between schizophrenia and the overall risk of lung cancer in populations of different races remains unknown due to the inclusion of predominantly European cohorts in this MR study. Second, the genetic association between schizophrenia and the overall risk of lung cancer found in this MR study was not verified by other databases due to certain limitations. Third, future studies should extensively investigate the potential mechanisms that elucidate the connection between smoking-related behaviors, schizophrenia, and overallrisk of lung cancer.
In conclusion, our bidirectional/multivariable and mediation MR study revealed a causal relationship between schizophrenia and the overall risk of lung cancer, with smoking serving as a significant mediator. Including smoking in the analysis reduced the explanatory power of schizophrenia for lung cancer risk, suggesting that smoking is a crucial causal factor in this relationship. This finding demonstrates a heightened risk of developing lung cancer among individuals with schizophrenia, highlighting the need to prioritize lung health in these patients. Therefore, early interventions to address smoking-related behaviors are essential to mitigate the increased risk of lung cancer in this population.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributionsXZ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. RY: Data curation, Writing – review & editing. XJ: Data curation, Writing – review & editing. JZ: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Zhejiang Provincial Medical and Health Technology Plan project (2024KY553).
AcknowledgmentsThe authors would like to thank the organizations for providing the GWAS data.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1367858/full#supplementary-material
Abbreviations95% CI, 95% confidence interval; GWAS, genome-wide association studies; IV, instrumental variables; IVW, inverse-variance weighted; LD, linkage disequilibrium; MR, Mendelian randomization; MR-Egger, Mendelian randomization-Egger; MR-PRESSO, MR Pleiotropy RESidual Sum and Outlier; OR, odds ratio; SNP, single nucleotide polymorphism.
References4. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. (2020) 126:134–40. doi: 10.1016/j.jpsychires.2019.08.002
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. (2017) 4:295–301. doi: 10.1016/S2215-0366(17)30078-0
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Nucifora FC Jr., Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol Dis. (2019) 131:104257. doi: 10.1016/j.nbd.2018.08.016
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. (2020) 50:2435–43. doi: 10.1017/S0033291719002678
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e78S–92S. doi: 10.1378/chest.12-2350
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Maneeton B, Maneeton N, Reungyos J, Intaprasert S, Leelarphat S, Thongprasert S. Prevalence and relationship between major depressive disorder and lung cancer: a cross-sectional study. Onco Targets Ther. (2014) 7:815–21. doi: 10.2147/OTT
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Gage SH, Jones HJ, Taylor AE, Burgess S, Zammit S, Munafò MR. Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci Rep. (2017) 7:40653. doi: 10.1038/srep40653
PubMed Abstract | CrossRef Full Text | Google Scholar
17. McFarland DC, Jutagir DR, Miller AH, Breitbart W, Nelson C, Rosenfeld B. Tumor mutation burden and depression in lung cancer: Association with inflammation. J Natl Compr Canc Netw. (2020) 18:434–42. doi: 10.6004/jnccn.2019.7374
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Zhu GL, Xu C, Yang KB, Tang SQ, Tang LL, Chen L, et al. Causal relationship between genetically predicted depression and cancer risk: a two-sample bi-directional mendelian randomization. BMC Cancer. (2022) 22:353. doi: 10.1186/s12885-022-09457-9
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. (2019) 22:343–52. doi: 10.1038/s41593-018-0326-7
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. (2022) 604:502–8. doi: 10.1038/s41586-022-04434-5
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. (2014) 46:736–41. doi: 10.1038/ng.3002
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Chen X, Kong J, Pan J, Huang K, Zhou W, Diao X, et al. Kidney damage causally affects the brain cortical structure: A Mendelian randomization study. EBioMedicine. (2021) 72:103592. doi: 10.1016/j.ebiom.2021.103592
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Yuan S, Mason AM, Carter P, Vithayathil M, Kar S, Burgess S, et al. Selenium and cancer risk: Wide-angled Mendelian randomization analysis. Int J Cancer. (2022) 150:1134–40. doi: 10.1002/ijc.33902
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Polityńska B, Pokorska O, Wojtukiewicz AM, Sawicka M, Myśliwiec M, Honn KV, et al. Is depression the missing link between inflammatory mediators and cancer? Pharmacol Ther. (2022) 240:108293. doi: 10.1016/j.pharmthera.2022.108293
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol. (2015) 25:1109–17. doi: 10.1016/j.euroneuro.2015.04.016
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Chen X, Kong J, Diao X, Cai J, Zheng J, Xie W, et al. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med. (2020) 9:9160–7. doi: 10.1002/cam4.3493
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med. (2003) 33:1357–67. doi: 10.1017/S0033291703008596
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Taipale H, Solmi M, Lähteenvuo M, Tanskanen A, Correll CU, Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. (2021) 8:883–91. doi: 10.1016/S2215-0366(21)00241-8
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Brown JS. Treatment of cancer with antipsychotic medications: Pushing the boundaries of schizophrenia and cancer. Neurosci Biobehav Rev. (2022) 141:104809. doi: 10.1016/j.neubiorev.2022.104809
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Ge F, Huo Z, Liu Y, Du X, Wang R, Lin W, et al. Association between schizophrenia and prostate cancer risk: Results from a pool of cohort studies and Mendelian randomization analysis. Compr Psychiatry. (2022) 115:152308. doi: 10.1016/j.comppsych.2022.152308
Comments (0)