The ureters are structures that surgeons need to pay close attention to during pelvic and abdominal surgeries. Iatrogenic ureteral injury is uncommon, but it can lead to serious complications, including loss of renal function (1). The ureters have a close anatomical relationship with the female reproductive system, and 41.9% of iatrogenic ureteral injuries occur during gynecological surgeries (2). The distal segment of the ureter is the most vulnerable, with over 80% of surgical injuries occurring in this anatomical region (3). In gynecological surgeries, ureteral injuries primarily occur during procedures such as hysterectomy and pelvic tumor surgeries (4) This is especially risky in complex and challenging pelvic surgeries involving conditions like deep Infiltrating endometriosis (DIE)-induced adhesions, large tumors, cervical fixation, and tumor infiltration. If ureteral injury is not promptly identified and repaired during surgery, the consequences are patient morbidity which could include ureteral obstruction, fistulas and irreversible kidney damage. Therefore, the prevention and early intraoperative detection of ureteral injury are crucial for patients’ outcomes. Ureteral stents have been used for intraoperative identification to help reduce iatrogenic ureteral injury. However, this practice increases surgical time and costs, and may lead to urinary complications such as infection, hematuria, and perforation (5, 6). Some studies have instead explored the use of radiopaque agents for ureteral identification. However, this technique exposes both patients and healthcare personnel to ionizing radiation and lacks real-time visual information, and hence is unable to provide immediate guidance during surgery (7). Another report has proposed the use of Indocyanine Green (ICG) to mark the ureteral branch of the uterine artery, enabling preservation of the ureteral branches during radical hysterectomy (8).
Methylene blue (MB) is both a dye and a fluorescent substance. During surgery, due to its blue staining characteristics, it is used to assess anastomotic leaks. With the development of near-infrared imaging systems, the near-infrared fluorescent properties of MB have been utilized for applications such as breast lymph node localization (9), vascular imaging, and bile duct imaging (10), all achieving favorable clinical results. After intravenous administration of MB, the majority undergoes renal metabolism and is ultimately excreted in the urine.
In this study, patients deemed to be prone to ureteral injury during surgery received intravenous MB and we utilized near-infrared fluorescence imaging technology to visualize ureters in gynecological surgeries. We also introduced the simultaneous use of ICG and MB for fluorescence imaging in cervical cancer and endometrial cancer surgeries, where ICG was used for visualizing sentinel lymph nodes (SLN) and MB was for ureteral imaging. This was our first instance of displaying two types of fluorescence using the same camera. And we conducted a preliminary assessment of the feasibility and safety of real-time ureteral localization using MB in gynecological surgeries. During the same period, some patients declined the use of MB, and the same surgeon proceeded to perform their surgeries without it. We preliminarily compared the adverse events between these patients and those who had received MB.
2 Material and methodsThis study was approved by the Institutional Review Board of the Guangxi Medical University Cancer Hospital. This study required patients to be over 18 years old and qualified for laparoscopic surgery for the removal of the uterus or pelvic lesions. Pregnant or lactating individuals, those with severe renal insufficiency, complete ureteral obstruction, G-6-PD deficiency, or known allergy to MB were excluded from this study. All patients provided signed informed consent before the surgery, and all these surgeries were performed by the same surgeon.
2.1 Fluorescence imaging equipmentDual-mode laparoscopic fluorescence image system (Guangdong OptoMedic, Guangdong, China) was used in this study. This device is capable of simultaneously exciting two near-infrared lights, allowing MB to display purple-blue fluorescence and ICG to display green fluorescence. The light source module uses 660 nm and 805 nm light as the excitation light for MB and ICG. During fluorescence imaging, the light source module simultaneously outputs white light illumination and near-infrared excitation light. The camera module captures both the reflected white light information from the observed tissue and the fluorescence information excited by the contrast agent, generating white light and fluorescence images. These images are processed by the image processing unit, and the final output includes white light, fluorescence, and composite images, providing fluorescence navigation information for the surgery.
The fluorescence imaging system offers three fluorescence modes: ICG fluorescence mode, MB fluorescence mode, and dual fluorescence mode (ICG and MB fluorescence). It can simultaneously display white light images and fluorescence images for each mode on the same screen.
2.2 Methods and assessmentAll surgeries were performed laparoscopically, and a dose of 1.5 mg/kg MB (Jiangsu Jichuan, Jiangsu, China) was added in 250 ml 5% glucose solution. At the beginning of surgeries, MB solution was rapidly infused intravenously. For cases requiring SLN imaging, such as cervical cancer and endometrial cancer, we additionally injected ICG solution (2.5 mg/ml) (Dandong Yichuang, Dandong, China) at the cervix's 3 and 9 o'clock positions after the instruments entered the abdominal cavity, with injection depths of 0.2 cm, 0.5 cm, and 1.0 cm, each side receiving 1 ml. During the surgeries, we observed both sides of the pelvic ureters in white light and fluorescence modes. The recommended observation position was the segment where the ureter crossed the iliac artery. Based on the patient's urine output and ureteral imaging, 20 mg of furosemide was administered intravenously if necessary to increase urine output. Concurrently, we monitored vital signs of patients, and compared renal function before and after surgeries. Adverse events after surgery were also collected.
3 ResultsWe included a total of 25 patients who underwent intraoperative ureteral MB fluorescence imaging at the Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, from February 2022 to December 2022. Among them, there were 10 cases of cervical cancer, 8 cases of endometrial cancer, 2 cases of deep infiltrating endometriosis (DIE), 3 cases of cervical uterine fibroids, and 2 cases of carcinoma of cervical stump. All patients underwent laparoscopic surgery, including radical surgery for cervical cancer with sentinel lymph node biopsy (SLNB), staging surgery for endometrial cancer with SLNB, lesion resection surgery for DIE, myomectomy for uterine fibroids, and radical trachelectomy. The basic clinical and surgical characteristics of the patients are listed in Table 1.
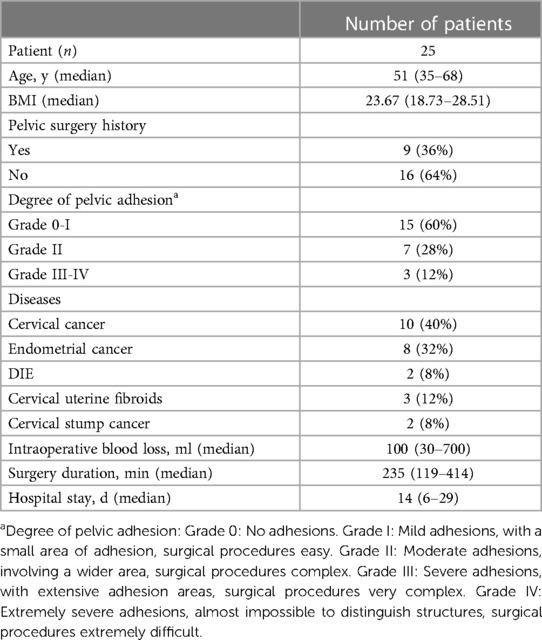
Table 1 Clinical and surgical data of patients.
3.1 Fluorescence imaging and assessmentWithin 15–20 min after the initiation of intravenous MB drip, a total of 45 fluorescent ureters were observed in 25 patients, 2 cases did not exhibit obvious fluorescence in both ureters throughout the surgery, and in 1 case, the right ureter became visible after the administration of furosemide, while the left side did not show clear fluorescence. The remaining cases showed fluorescence in both ureters, and the visibility rate was 90%. We found that even though the ureters were covered by peritoneum, the ureters’ fluorescence was still visible. Upon opening the peritoneum, the location of the fluorescent ureters corresponded to the actual anatomy. Prior to opening the peritoneum and freeing the ureters, approximately 15 ureters were visible under white light (30%), whilst the rest were indistinct or invisible. In cases of DIE and cervical myoma, the ureters were clearly imaged, as shown in Figures 1, 2. And in cervical and endometrial cancer cases, both the SLN with green fluorescence and the ureters with violet-blue fluorescence were visible simultaneously, as shown in Figure 3. Among cases which both ureters exhibited fluorescence, 2 cases had a more pronounced fluorescence in the left ureter, 1 case had a more pronounced fluorescence in the right ureter. These three cases had varying degrees of ureteral incomplete obstruction and hydronephrosis due to lesion compression, and the affected side showing more pronounced fluorescence. It was observed that in cases with severe ureteral incomplete obstruction and hydronephrosis, the fluorescence persisted, while in cases without ureteral hydronephrosis, the fluorescence followed the flow of urine. And when the ureteral obstruction was relieved, fluorescence flows with the urine (Supplementary Videos 1, 2, Supplementary Video 1 shows fluorescence imaging in the surgery of cervical leiomyoma with ureteral incomplete obstruction and hydronephrosis, Supplementary Video 2 shows fluorescence imaging in the surgery of cervical cancer with normal ureters). The fluorescence image of the ureters could be observed as early as 5 min after the intravenous administration of MB, and the longest duration lasted for 4 h and 10 min. In this study, ureteral fluorescence was still observable in 16 cases (64%) at the conclusion of the surgery.
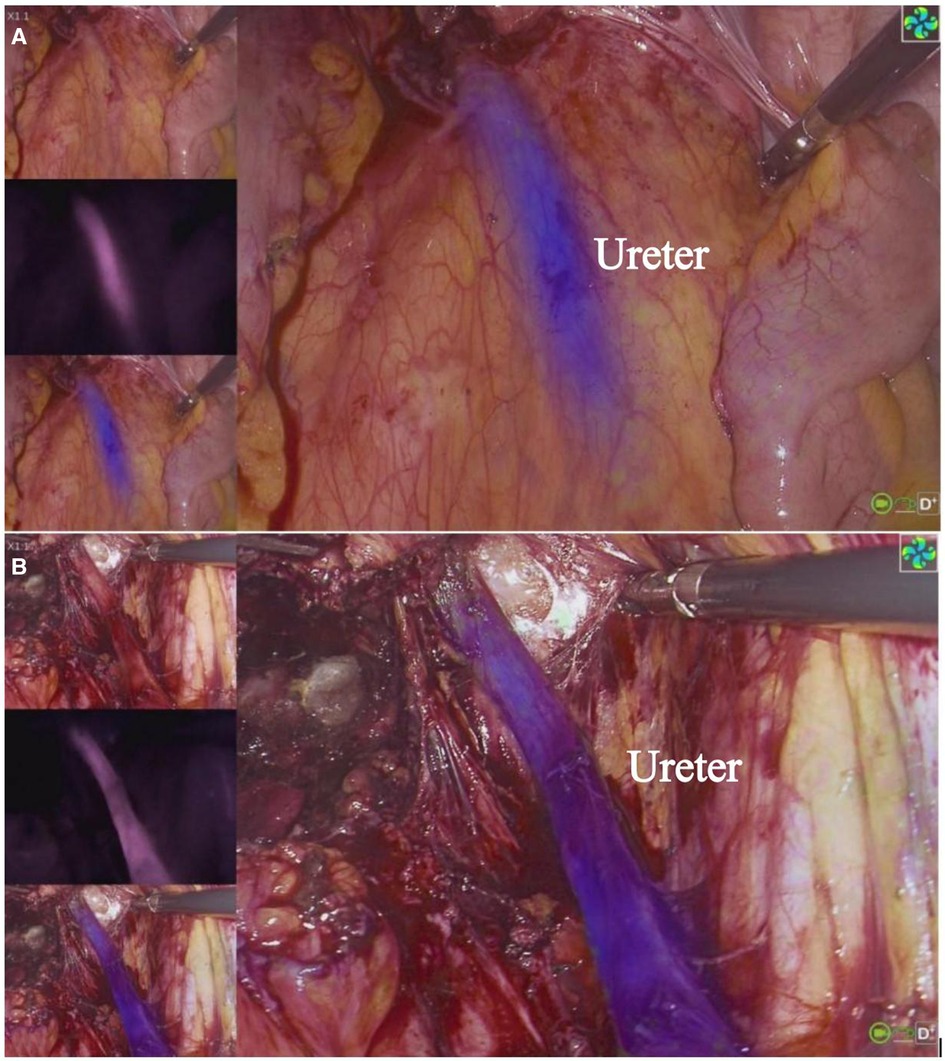
Figure 1 Visualization of ureter with MB in DIE. The three images on the left, from top to bottom, are white light image, fluorescent only black and white image and overlay of fluorescence (blue) to the white light image. The main screen displays the overlay image. In (A), the ureter is covered by the peritoneum and cannot be clearly observed under white light, and (B) shows the ureter can be clearly observed under both white light and fluorescence modes after it has been freed, and the position is consistent with the location shown in (A).

Figure 2 Visualization of ureter during laparoscopic removal of cervical leiomyoma.
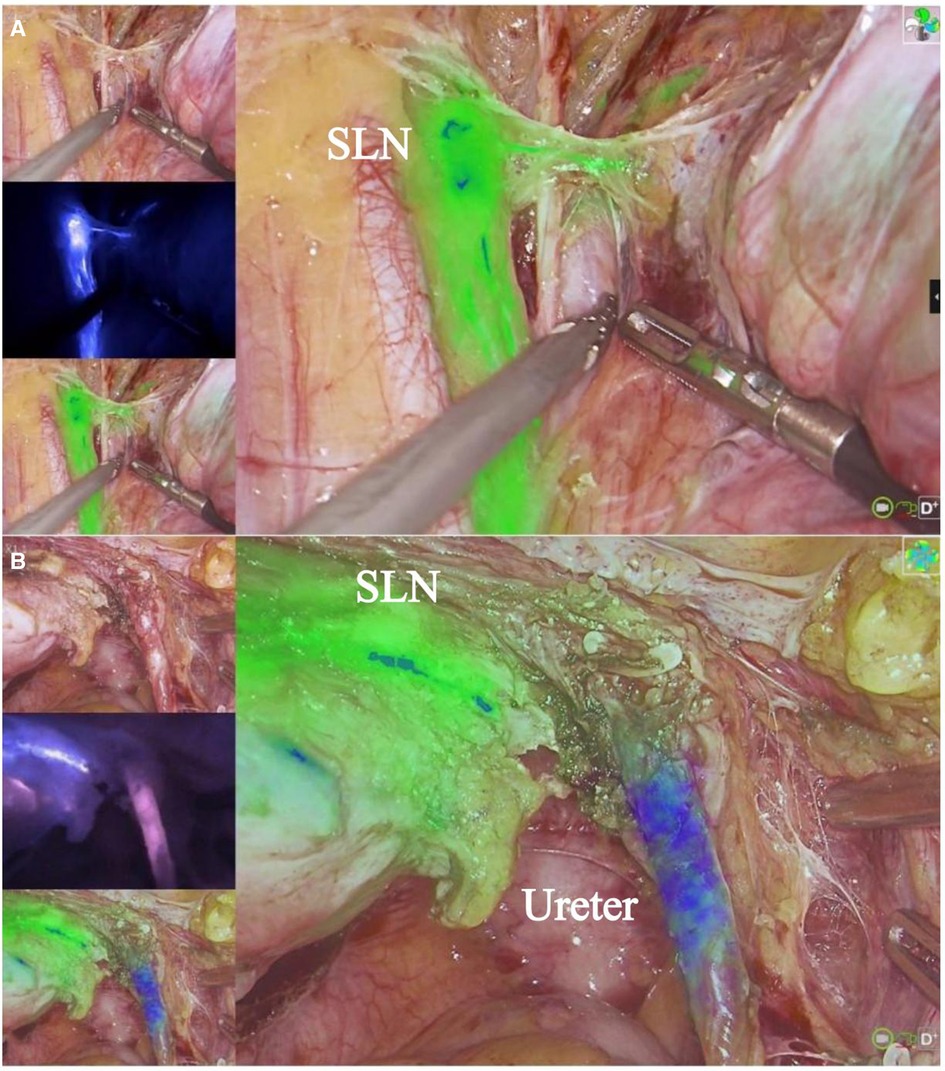
Figure 3 Radical surgery for cervical cancer and SLN. (A) shows ICG imaging of SLN. (B) shows MB imaging of ureter. The imaging system can simultaneously display both ICG and MB fluorescence.
3.2 Adverse eventsDuring surgeries, we observed 2 cases (8%) of decreased oxygen saturation, with the lowest level reaching 95%. After increasing the oxygen supply, the saturation quickly recovered. Throughout the surgical procedures, no adverse events related to MB occurred, such as hypotension, increased heart rate, arrhythmias, hemolysis, or allergies. No iatrogenic ureteral injuries, vascular or nerve damage, or other surgery-related complications occurred during surgeries (0%). Postoperatively, all patients had blue-green urine, which gradually subsided. There were no cases of renal function impairment or mortality after surgeries. We closely monitored potential postoperative adverse reactions which were possibly related to MB, including abdominal pain, distension, allergies (itching, etc), dizziness, headaches, nausea, vomiting, cardiovascular symptoms (chest tightness, palpitations, hypotension, arrhythmias), etc. 6 patients(24%) experienced the adverse reactions of grade 1 and 2, according to the Clavien-Dindo Classification (11). From February 2022 to December 2022, the same surgeon performed 78 laparoscopic procedures without the use of MB as patients had declined the intraoperative use of MB (Group B). The indications for surgery were radical cervical cancer surgery, endometrial cancer staging surgery, DIE lesion removal surgery, myomectomy, and hysterectomy. Ureteral injury occurred in 1 case (1.3%) due to dense adhesions at the distal end of the ureter, and a double-J stent was placed during the surgery. Postoperatively, 21 cases (26.9%) experienced the aforementioned adverse reactions of grade 1 and 2. Details of adverse events are presented in Table 2.

Table 2 Adverse events.
4 DiscussionIn gynecological surgeries, iatrogenic ureteral injury rates can be as high as 1.5% (12). Traditionally, common ureteral stents (13), luminescent ureteral stents (5), ureteral fluorescence stents (14–16), or intravenous injection of radioactive substances (7) were used for ureteral localization. However, these methods have disadvantages such as infection or ionizing radiation. MB is cheap, and by using near-infrared fluorescence imaging technology, patient's ureter appears purple-blue. This technique enables real-time, in vivo visualization of the ureter's location without ionizing radiation, making it non-invasive. Ureters covered by the peritoneum could not be immediately identified under white light but were clearly visualized in fluorescence mode. In our study, 90% of the ureters were clearly visualized in fluorescence mode, which is significantly higher than in white light mode. So, in fluorescence mode, the ureters were more easily detected, which shortened the surgical time for ureteric localization and helped avoid ureteral injury. Direct visualization of the ureter facilitates ureterolysis and helps to minimise direct trauma, delayed thermal injury and damaging the inferior hypogastric nerve plexus which tends to run below the ureter. Additionally, it can assist surgeons in quickly identifying abnormal, variant, displaced, or ureters affected by adhesions and fibrosis due to multiple surgeries or pelvic radiotherapy. In this study, during the resection of pelvic lesions in patients with DIE, MB-enhanced ureteral visualization and clearly delineated its boundaries, aiding lesion removal. Patients with large cervical fibroids, which compressed one side of the ureter causing local displacement, underwent ureteral fluorescence imaging with intravenous MB, revealing a distinct purple-blue colour that facilitated observation of the ureter's course and prevented ureteral injury.
Currently, there is no standard consensus on the dosage for MB, and there is a slight dose discrepancy on the optimal MB dose according to various studies. Verdeek (17)reported that in open surgeries for cervical cancer and ovarian cancer, an dosage of 0.25 mg/kg was recommended. In studies related to laparoscopic or open colorectal surgeries, Polom (12) suggested a dosage of 0.5 mg/kg and Barnes TG (18) recommended 0.75 mg/kg, whilst Yeung (19) recommended 1 mg/kg. Our study found that in gynecological surgeries, the intravenous rapid infusion of 1.5 mg/kg of MB resulted in clear visualization of ureters without associated safety issues. However, further research is needed to determine the ideal dosage. Almost all ureters could be observed in 15–20 min after administration of MB. Therefore, our experience suggests that the optimal observation time is 15–20 min after intravenous MB infusion. We also observed that at this dosage, the fluorescence imaging duration of MB was relatively long, with over half of the cases still showing ureteral imaging at the end of surgeries. This prolonged visualization allowed surgeons to confirm whether ureteral injuries had occurred.
MB is metabolized by the kidneys and excreted in the urine, limiting its use in individuals with severe renal impairment. Additionally, ureteral peristalsis causes pulsatile urine flow, affecting the fluorescence signal which varies over time (20). This study revealed that in cases of incomplete ureteral obstruction, the severity of hydronephrosis is positively correlated with the intensity of MB fluorescence imaging, whilst slow peristalsis or reduced urine volume led to unclear imaging. We also found that the clarity of imaging could be improved by injecting one or two boluses of Furosemide (20 mg) during surgeries. However, a study by Matsui (21) reported that when diuretics were used in combination with MB, increased urine excretion did not affect the fluorescence of MB dye. Currently, there is also no standard for the use of diuretics, dosage, or related issues. Further research is clearly needed to clarify these matters.
In our study, some patients did not have ureteral peristalsis or urine volume problems, but resulting in weak or unclear imaging. This phenomenon might be associated with fluorescence quenching or renal metabolism. When using the fluorescence laparoscope's gain function, the majority of ureters became more clearly visualized. Therefore, when encountering such situations during surgery, using the gain function may be effective. A small number of ureters were not visualized during the surgery. Review of these cases found that the reasons for this included obesity, increased visceral fat, thickened tissues, and poor ureteral activity with low urine volume. For these patients, our subsequent research may explore whether increasing the dosage of MB or using diuretics can improve visualization.
Our study found that MB is effective and safe for visualizing ureters in gynecological laparoscopic surgery. There were no iatrogenic ureteral injuries during surgeries involving MB, and in contrast, similar surgeries performed by the same surgeon during the same period without using MB had a 1.3% rate of ureteral injury. The reported incidence of iatrogenic ureteral injuries in gynecological surgeries is 0.15–1.5% (22). We preliminary propose that ureteral MB imaging can reduce the occurrence of iatrogenic ureteral injury in gynecological surgeries. The incidence of postoperative complications was similar between these two groups. We consider that the “MB-related adverse reactions” observed postoperatively might be attributed to surgical and anesthetic factors rather than an apparent association with MB. We acknowledge that our study has limitations, due to the small sample size and its short duration, therefore further clinical research is needed to validate its efficacy and safety.
We summarized previous studies on intraoperative ureteral visualization with either ICG or MB (Table 3). The studies indicated that using ICG for ureter visualization had a success rate of 100%, but it required the assistance of ureteral catheters. And in colorectal surgeries, with intravenous MB, the overall ureteral visibility rate ranged from 50% to 92.8%, with good safety. However no definitive clinical recommendations or standards have been established yet. Our study is the only one to report the use of MB for fluorescent ureteral imaging in gynecological laparoscopic surgeries, and has achieved satisfactory results.
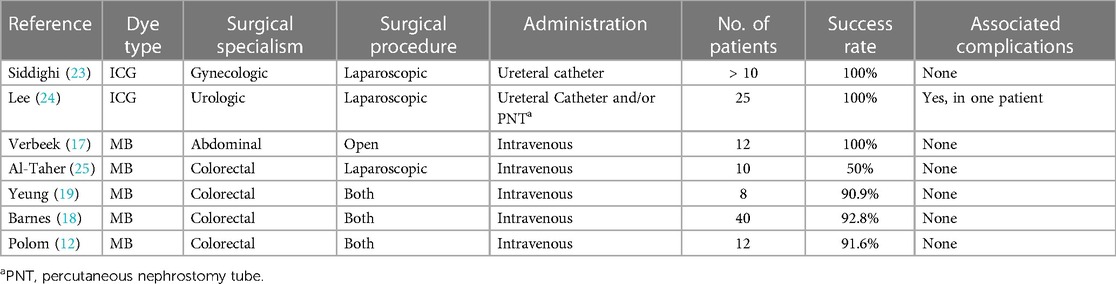
Table 3 Previous studies of intraoperative ureteral visualization.
We believe this technique can be widely applied in future gynecological laparoscopic surgeries. Moreover, our study explored, for the first time, the dual-dye technique of ureteral MB fluorescence imaging (for the ureters) along with ICG fluorescence imaging for sentinel lymph nodes (SLN) in cervical or endometrial cancer, using the same camera system simultaneously, and this shows promising results. This dual imaging technique holds significant promise for patients requiring SLN biopsy and mapping, allowing surgeons to precisely remove lymph nodes while simultaneously avoiding ureteral damage. We are currently conducting a prospective clinical trial, and we expect to obtain more comprehensive results and clinical recommendations in the future.
5 ConclusionsOur initial study presents the application of ureteric MB fluorescence in gynecological surgeries. We visualized two fluorophores simultaneously through one camera system for the first time and achieved favorable outcomes. Our research demonstrates that intravenous infusion of MB can facilitate laparoscopic visualization of the ureters in gynecological surgeries under non-invasive and radiation-free conditions. This technique reduces surgical complexity, particularly benefiting less experienced surgeons by significantly minimizing the risk of ureteral injury and decreasing overall surgical duration. Furthermore, our initial experience is that this technique does not increase the risk of intraoperative or postoperative adverse events, indicating the safety of this technology. However, there is currently no clear consensus on the optimal dosage of MB or the use of diuretics. These questions necessitate further clinical investigation.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Guangxi Medical University Cancer Hospital Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsRS: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. FS: Data curation, Investigation, Writing – original draft. HS: Conceptualization, Methodology, Supervision, Writing – review & editing. XQ: Data curation, Visualization, Writing – review & editing. DY: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. YL: Data curation, Writing – review & editing. HW: Resources, Writing – review & editing. YW: Visualization, Writing – review & editing. XC: Resources, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by Natural Science Foundation of Guangxi (no. 2021JJA140907) and Guangxi Zhuang Autonomous Region Clinical Key Specialized Subject Construction project funds.
AcknowledgmentsWe appreciate all the contributions that supported this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2024.1387038/full#supplementary-material
Supplementary Video 1
MB fluorescence imaging of ureter in the surgery of cervical leiomyoma with ureteral incomplete obstruction and hydronephrosis.
Supplementary Video 2
MB fluorescence imaging of ureter in the surgery of cervical cancer with normal ureters.
1. Yanagisawa T, Mori K, Quhal F, Kawada T, Mostafaei H, Laukhtina E, et al. Iatrogenic ureteric injury during abdominal or pelvic surgery: a meta-analysis. BJU Int. (2023) 131(5):540–52. doi: 10.1111/bju.15913
PubMed Abstract | Crossref Full Text | Google Scholar
2. Li X, Yang K, Ding G, Zou X, Ye L, Wu J, et al. Etiology, characteristics and management of ureteric injury: experience from a nationwide study. Transl Androl Urol. (2022) 11(6):794–802. doi: 10.21037/tau-21-998
PubMed Abstract | Crossref Full Text | Google Scholar
3. Zilberman DE, Rimon U, Morag R, Winkler HZ, Ramon J, Mor Y. Non-surgical treatment of latrogenic postoperatively diagnosed ureteral injuries. Isr Med Assoc J. (2015) 17(4):227–30. PMID: 26040048
PubMed Abstract | Google Scholar
4. Aguilera A, Rivas JG, Quintana Franco LM, Quesada-Olarte J, Carrion DM, Martínez-Piñeiro L. Ureteral injury during abdominal and pelvic surgery: immediate versus deferred repair. Cent European J Urol. (2019) 72(3):312–8. doi: 10.5173/ceju
PubMed Abstract | Crossref Full Text | Google Scholar
5. Boyan WP Jr, Lavy D, Dinallo A, Otero J, Roding A, Hanos D, et al. Lighted ureteral stents in laparoscopic colorectal surgery; a five-year experience. Ann Transl Med. (2017) 5(3):44. doi: 10.21037/atm.2017.02.01
PubMed Abstract | Crossref Full Text | Google Scholar
7. Berland TL, Smith SL, Metzger PP, Nelson KL, Fakhre PG, Chua HK, et al. Intraoperative gamma probe localization of the ureters: a novel concept. J Am Coll Surg. (2007) 205(4):608–11. doi: 10.1016/j.jamcollsurg.2007.04.017
PubMed Abstract | Crossref Full Text | Google Scholar
8. Long Y, Yao Y, Yao DS. Indocyanine green angiography for preserving the ureteral branch of the uterine artery during radical hysterectomy: two case report. Medicine (Baltimore). (2018) 97(40):e12692. doi: 10.1097/MD.0000000000012692
PubMed Abstract | Crossref Full Text | Google Scholar
9. Agrawal SK, Hashlamoun I, Karki B, Sharma A, Arun I, Ahmed R. Diagnostic performance of indocyanine green plus methylene blue versus radioisotope plus methylene blue dye method for sentinel lymph node biopsy in node-negative early breast cancer. JCO Glob Oncol. (2020) 6:1225–31. doi: 10.1200/GO.20.00165
PubMed Abstract | Crossref Full Text | Google Scholar
10. Tuysuz U, Aktas H, Batı IB, Emıroglu R. The role of intraoperative cholangiography (IOC) and methylene blue tests in reducing bile leakage after living donor hepatectomy. Asian J Surg. (2021) 44(1):147–52. doi: 10.1016/j.asjsur.2020.04.001
PubMed Abstract | Crossref Full Text | Google Scholar
11. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
PubMed Abstract | Crossref Full Text | Google Scholar
12. Polom W, Migaczewski M, Skokowski J, Swierblewski M, Cwalinski T, Kalinowski L, et al. Multispectral imaging using fluorescent properties of indocyanine green and methylene blue in colorectal surgery-initial experience. J Clin Med. (2022) 11(2):368. doi: 10.3390/jcm11020368
PubMed Abstract | Crossref Full Text | Google Scholar
13. Kovachev SM, Kovachev MS. The role of perioperative ureteral stenting for urologic complications in radical surgery of cervical cancer. Urologia. (2021) 88(4):348–54. doi: 10.1177/03915603211001178
PubMed Abstract | Crossref Full Text | Google Scholar
14. Hamada M, Matsumi Y, Sekimoto M, Kurokawa H, Kita M, Kinoshita H. Image navigation surgery with the fluorescent ureteral catheter of recurrent tumors in the pelvic cavity. Dis Colon Rectum. (2022) 65(2):e72–6. doi: 10.1097/DCR.0000000000002144
PubMed Abstract | Crossref Full Text | Google Scholar
15. Kisu I, Iida M, Shiraishi T, Iijima M, Nakamura K, Matsuda K, et al. Real-time intraoperative ureter visualization with a novel near-infrared ray catheter during laparoscopic hysterectomy for gynecological cancer. J Gynecol Oncol. (2021) 32(6):e93. doi: 10.3802/jgo.2021.32.e93
PubMed Abstract | Crossref Full Text | Google Scholar
16. Barberio M, Al-Taher M, Felli E, Ashoka AH, Marescaux J, Klymchenko A, et al. Intraoperative ureter identification with a novel fluorescent catheter. Sci Rep. (2021) 11(1):4501. doi: 10.1038/s41598-021-84121-z
PubMed Abstract | Crossref Full Text | Google Scholar
17. Verbeek FP, van der Vorst JR, Schaafsma BE, Swijnenburg RJ, Gaarenstroom KN, Elzevier HW, et al. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: a first in human experience. J Urol. (2013) 190(2):574–9. doi: 10.1016/j.juro.2013.02.3187
PubMed Abstract | Crossref Full Text | Google Scholar
18. Barnes TG, Hompes R, Birks J, Mortensen NJ, Jones O, Lindsey I, et al. Methylene blue fluorescence of the ureter during colorectal surgery. Surg Endosc. (2018) 32(9):4036–43. doi: 10.1007/s00464-018-6219-8
PubMed Abstract | Crossref Full Text | Google Scholar
19. Yeung TM, Volpi D, Tullis ID, Nicholson GA, Buchs N, Cunningham C, et al. Identifying ureters in situ under fluorescence during laparoscopic and open colorectal surgery. Ann Surg. (2016) 263(1):e1–2. doi: 10.1097/SLA.0000000000001513
PubMed Abstract | Crossref Full Text | Google Scholar
20. Zocola E, Meyer J, Christou N, Liot E, Toso C, Buchs NC, et al. Role of near-infrared fluorescence in colorectal surgery. World J Gastroenterol. (2021) 27(31):5189–200. doi: 10.3748/wjg.v27.i31.5189
PubMed Abstract | Crossref Full Text | Google Scholar
21. Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, et al. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery. (2010) 148(1):78–86. doi: 10.1016/j.surg.2009.12.003
PubMed Abstract | Crossref Full Text | Google Scholar
22. Cwalinski T, Polom W, Marano L, Roviello G, D’Angelo A, Cwalina N, et al. Methylene blue-current knowledge, fluorescent properties, and its future use. J Clin Med. (2020) 9(11):3538. doi: 10.3390/jcm9113538
PubMed Abstract | Crossref Full Text | Google Scholar
25. Al-Taher M, van den Bos J, Schols RM, Bouvy ND, Stassen LP. Fluorescence ureteral visualization in human laparoscopic colorectal surgery using methylene blue. J Laparoendosc Adv Surg Tech A. (2016) 26(11):870–5. doi: 10.1089/lap.2016.0264
Comments (0)