Coronavirus Disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 (1) and led to approximately 7 million confirmed deaths in both pediatric and adult populations (2). The clinical presentation of COVID-19 varies, ranging from asymptomatic cases to those involving multiple organs (3). Compared to adults, children generally experience a lower incidence, milder symptoms, and reduced mortality rate (3–9). Common symptoms in children include fever and cough, with additional manifestations such as shortness of breath, sore throat, headache, dizziness, myalgia, and gastrointestinal symptoms (6, 10, 11).
Some pediatric patients, especially infants and toddlers, may require hospitalization and face a higher risk of complications and mortality (7, 10). Certain underlying medical conditions are associated with more severe symptoms and outcomes (12). Children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are vulnerable to severe complications, including acute respiratory distress syndrome, myocarditis, acute renal and multi-organ failure, and multisystem inflammatory syndrome in children (MIS-C) (13). MIS-C represents a hyperinflammatory state linked to SARS-CoV-2 infection, posing a significant risk of morbidity and mortality (6). It's noteworthy, however, that the overall mortality rate among children remains relatively low, as indicated by a meta-analysis reporting a rate of 2.4 percent (13, 14).
In Iran, over 140,000 deaths related to COVID-19 have been reported in general population (11). However, the actual number of COVID-19 cases is likely higher (15). Numerous studies have described the clinical characteristics of children with COVID-19 in Iran since the outbreak began (7, 16–23). In a comprehensive report covering 18 months of the epidemic and 20,506 admitted patients, intensive care unit (ICU) admission, ventilator use, and total mortality rates were reported at 14.5%, 5.7%, and 2.9% of the cases, respectively (7). Another multicenter study involving 645 patients reported a mortality rate of 2.5%, primarily among individuals with comorbidities and dyspnea (18).
The vaccination rates for COVID-19 are remarkably lower in children, with many countries refraining from recommending vaccines for those under 5 years old (24, 25). Moreover, the risk of infectious disease transmission, including COVID-19, is significantly heightened within densely populated pediatric settings such as schools. In response to these concerns, this multicenter and retrospective study was conducted to analyze the demographic and clinical characteristics of children with COVID-19 in Mazandaran province. Furthermore, our study aimed to evaluate the case severity and identify risk factors influencing ICU admissions.
2 Materials and methods 2.1 Study population and settingThis multicenter observational study was performed on children aged 0–21 years with probable or confirmed COVID-19 infection diagnosis. The patients were referred from the selected referral hospitals of 17 counties of Mazandaran province, northern Iran, from 19 Feb to 14 Aug 2021.
2.2 Diagnostic methodsThe diagnosis of COVID-19 was based on a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) kit or rapid test through nasal, oropharyngeal, or nasopharyngeal swab specimens. All COVID-19 RT-PCR or rapid tests were performed in hospitals or previously determined health centers in each county. Probable cases were identified through lung CT scan findings or clinical symptoms, along with exposure to individuals confirmed to have COVID-19.
2.3 Severity classificationAccording to the WHO guidelines (26, 27; Supplementary Data Sheet 1), patients were defined into four groups of mild, moderate, severe and critical cases. The disease severity was defined as follows:
• Mild: Mild clinical symptoms with no radiographic finding compatible with pneumonia,
• Moderate: Fever, respiratory symptoms, and radiographic findings compatible with pneumonia with <30% pulmonary involvement,
• Severe: One of the following factors: respiratory distress (retraction, grunting, central cyanosis, inability to express complete sentences, etc.), tachypnea (respiratory rate ≥30/min); hypoxemia; needing supplemental oxygen and oxygen saturation (SpO2) lower than 94% in the air room; >50% pulmonary involvement,
• Critical: Acute respiratory distress syndrome (ARDS), septic shock, Multisystem inflammatory syndrome in children (MIS-C), and needing mechanical ventilation.
2.4 Data collectionPatients with COVID-19 were both inpatients and outpatients. Mild cases included outpatients visited by pediatricians or pediatric infectious diseases subspecialists in clinics in whom no laboratory test or chest CT scan was performed. Moderate, severe and clinical cases were inpatients admitted to mentioned hospitals and demographic and clinical characteristics and COVID-19 diagnostic test data was extracted from their medical records in addition to the COVID-19 registry system of Mazandaran University of Medical Sciences.
2.5 Statistical analysisResults were presented as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables and frequency with percentage for categorical variables in each group. According to the Kolmogorov-Smirnov test, the distribution of quantitative variables was non-normal; So, the Kruskal-Wallis test was used for intergroup comparison. Univariate and multivariate logistic regression Cox proportional hazard models were used to determine the effective factors on disease severity during their duration of hospitalization for COVID-19 inpatients. Statistical analysis was performed by using Statistical Package for the Social Sciences (SPSS) software, version 22.0. P-values less than 0.05 were considered statistically significant.
3 Results 3.1 Demographics and BMI distributionThis study included 1,031 children diagnosed with COVID-19, among whom 61 were MIS-C patients. The number of mild, moderate and severe/critical patients was 156, 671, and 204, respectively. The median age of the patients was 6 years [interquartile Range (IQR): 1–14], which was 11 years in the mild group and 4 years in the moderate and severe/critical groups. The age distribution across the three groups showed a statistically significant difference (P < 0.001). Also, 6% of the patients were aged under 1 month, 38.8% were between 1 month to 5 years, and 55.19% were older than 5 years. Overall, 54% of the patients were male, and 58.29% resided in urban areas. 11.44% of the children had underlying diseases, with a higher prevalence in the severe/critical group (22.06%), showing a significant difference compared to the other two groups (P < 0.001). Body mass index (BMI) information was available for half of the patients (50.82%), among whom 17.55% were underweight, 46.18% were normal, and 36.25% were overweight/obese. It's worth noting that none of the cases had severe failure to thrive (FTT) in this study. About 50% of the patients in the severe/critical group were overweight/obese, compared to 28.57% and 35.11% in the mild and moderate groups, respectively (P < 0.001). 42.77% reported a history of close contact with confirmed cases with COVID-19 (Table 1).
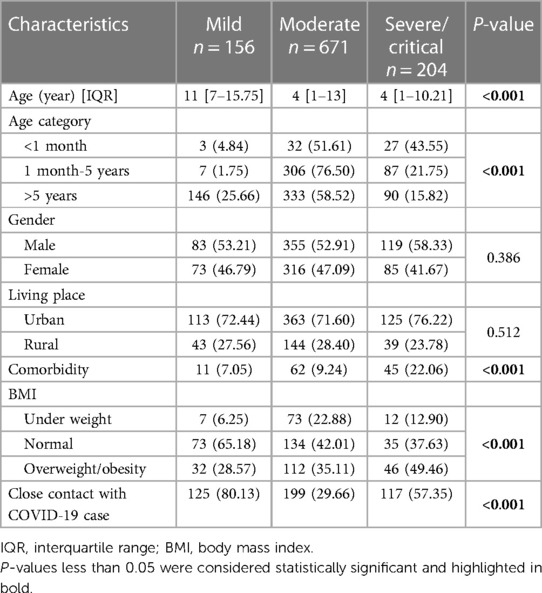
Table 1 Characteristics of children with COVID-19 based on the severity of the disease.
A lower prevalence of patients with a normal BMI was observed in the inpatient group compared to the outpatient group (40.92% vs. 65.77%), and this difference was statistically significant (P < 0.001) (Table 2). Other characteristics and outcomes based on the two groups of outpatients and inpatients are presented in Supplementary Table 1.
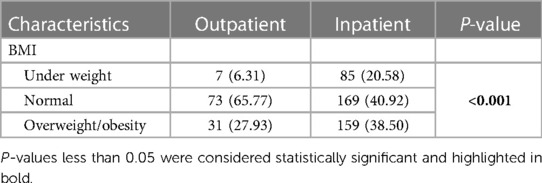
Table 2 Frequency of BMI in outpatients and inpatients.
3.2 Symptoms, treatment, and outcomesIn terms of symptoms in children, fever was the most common (71.97%), followed by cough (34.43%), dyspnea (24.83%), weakness (22.79%), and nausea/vomiting (21.92%). The mild group showed a lower prevalence of respiratory distress, weakness, and skin rash, compared to others (P < 0.05). Approximately 25.70% and 9.50% of the patients received antibiotics and corticosteroids, respectively. The corticosteroid use was significantly higher in the severe/critical group compared to the moderate group (P < 0.001). 5.24% of all patients had oxygen saturation levels below 93%, and 15.42% required oxygen therapy. Within the inpatient group, 173 children (19.77%) required ICU admission, and unfortunately, 8 children (0.91%) succumbed to the illness which among them 3 patients were MIS-C (Table 3).
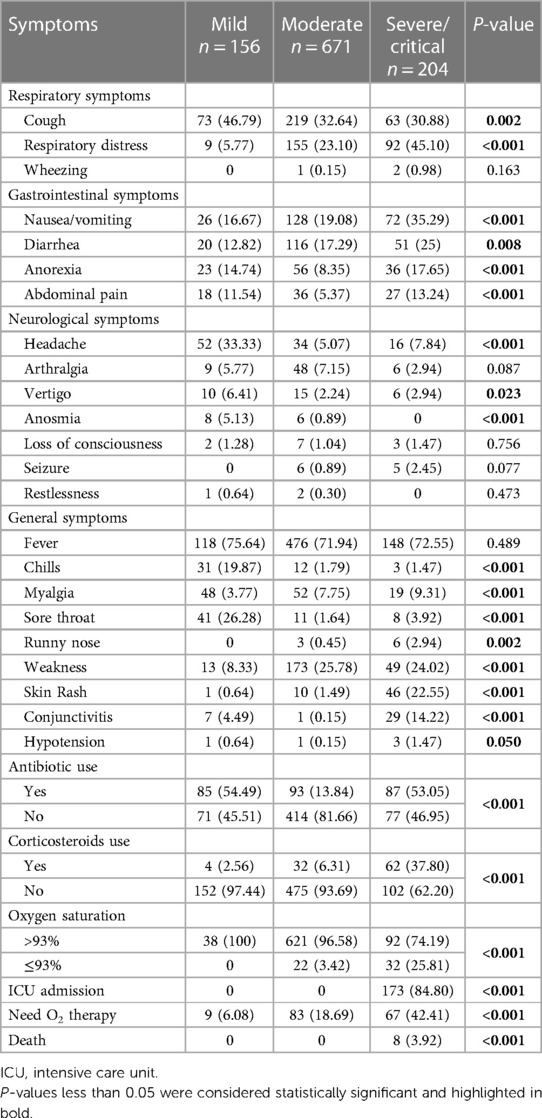
Table 3 Symptoms and outcomes of children with COVID-19 based on the severity of the disease.
3.3 Risk factors for ICU admissionLogistic regression analysis was conducted on inpatient children diagnosed with COVID-19. Clinical factors that exhibited statistical significance with a P-value <0.3 in the single-factor analysis were included in the multifactorial model. The results showed that children with underlying diseases, gastrointestinal symptoms, and obesity were 4.16, 3.10 and 2.17 times more likely for ICU admission than other hospitalized children (Table 4).
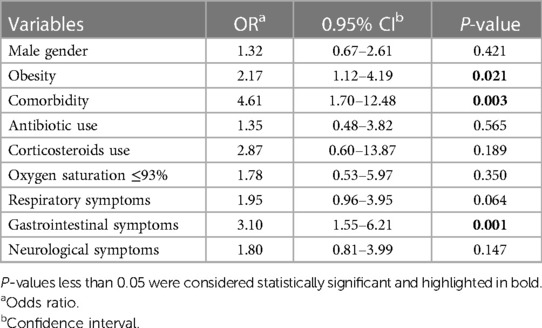
Table 4 The effective factors on ICU admission for COVID-19 by multivariate logistic regression.
3.4 Risk factors for disease severityThe result of the Cox regression model in hospitalized children with COVID-19 has been presented in Table 5. Variables with P < 0.3 in single-variable analysis were entered into the multivariable model. Results revealed that the variables of ICU admission and the need for oxygen therapy increase the hazard of disease severity by 9.95 and 2.34 times, respectively (P < 0.001) (Table 5).
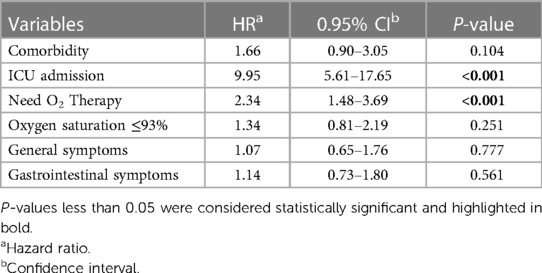
Table 5 Determining the effective factors on disease severity for COVID-19 inpatients, using multivariate cox regression.
4 DiscussionIn this study, the demographic and clinical presentations of confirmed or probable pediatric COVID-19 were reported. These patients sought medical attention over six months, coinciding with the second and third waves of the pandemic in Iran. During these waves, variants such as B.1.1.7 (Alpha in the WHO classification), B.1.351 (Beta), and B.1.617.2 (Delta) were predominant, while more recent variants like Omicron had not yet emerged. The Delta variant likely led to a higher pneumonia rate, more severe disease, and increased mortality compared to other variants. However, analysis of laboratory test results did not show significant differences between variants in children (17, 28). None of the children were vaccinated during this period, but vaccination programs had been initiated for adults.
The median age of our patients was 6 years. The majority of children with COVID-19 in our study were mild to moderate, aligning with findings from other research studies (29–33). With increasing age, the hospitalization and the severity of the disease decreased. Nevertheless, approximately half of our hospitalized patients were over 5 years old. This finding is consistent with previous studies (16, 22, 34). Male gender was slightly more prevalent (1.17:1), and its frequency was a bit higher in the severe/critical group. Similar ratios have been observed in some other studies and across newborns and adults (3, 9, 16, 34–38).
Along with other studies, fever and cough were the most prevalent symptoms in our study (4, 16, 36, 39–41). Contrary to previous reports, fever was significantly more prominent in our study (37, 42, 43). In some studies, including one involving 6,610 hospitalized children with COVID-19 in Iran, dyspnea was reported as the third common symptom, supporting our findings (4, 37, 44).
After adjusting for the impact of other factors, the presence of underlying diseases, gastrointestinal symptoms, and obesity significantly increased the risk of ICU admission by 4.16, 3.10 and 2.17 times, respectively. Notably, besides ICU admission and the need for oxygen therapy, none of the other factors emerged as a risk factor for the severity of the disease. Assessing factors influencing mortality was challenging due to the limited number of fatalities.
In our study, although the specific details of underlying medical conditions (aside from abnormal BMI) were not provided, the presence of such conditions showed a significant association with the development of severe or critical disease. Furthermore, having an underlying medical condition independently increased the risk of ICU admission by more than 4-fold. This finding aligns with some prior studies, consistently highlighting underlying medical conditions as an established risk factor (36, 45).
Obesity is a known risk factor for COVID-19 (45–47). In this study, data on height and weight were available for half of the patients, in which 36.25% had a BMI above the normal range. The majority of the mild (outpatient) cases had normal BMI. O'Neill et al. reported obesity as the most significant risk factor for hospitalization, followed by diabetes (45). A lower BMI correlated with increased hospitalization, appears to have no association with the severity of the disease. One possible explanation for the impact of a low BMI was observed in the study by Crespo et al., where circulating CD3, CD4 and B lymphocytes were found to be lower in underweight children (41).
Among hospitalized patients, the ICU admission and mortality rates were 19.77% and 0.91%, respectively. Large reports from Iran indicated a significantly higher mortality rate in children, with inpatient mortality of 2.9%–5.3% in various studies (7, 37). Moreover, ICU admission rates in these studies were 14.5% and 13%, which are lower compared to our study (7, 37). Apart from methodological differences, this variation might stem from a better understanding of the presentation and management of the new disease one year after its emergence. Moreover, a well-established referral system existed in the province, facilitating the identification and transfer of severe or critical cases to referral centers. In these centers, the threshold for ICU admission was lower, and patients were admitted to the ICU earlier if possible.
In our hospitalized patients, 61 (6.97%) were diagnosed with MIS-C, constituting 37.5% (3 individuals) of our overall mortality. In a systematic review, 11.28% of examined children exhibited symptoms consistent with MIS-C (48). However, a single-center long-term study in Mexico reported a 0.6% prevalence of MIS-C (36). Therefore, MIS-C is recognized as one of the most severe consequences/presentations of COVID-19, demanding vigilant attention from the healthcare team. In our study, 4.91% of MIS-C patients succumbed to the illness. A similar study in Iran reported a 5.99% mortality rate in children, indicating comparable findings (3).
4.1 LimitationsOur study faced several limitations. First, due to insufficient precision in categorizing cases based on waves, we were unable to determine which symptoms were more common in each wave. Additionally, a significant portion of cases were obtained from the registry systems, which restricted our access to laboratory analysis results and imaging data. Moreover, the predominance of moderate and severe/critical patients in our study, stemming from limited participation among mild cases, compromised our ability to assess prevalence accurately. These limitations should be considered when interpreting the findings of our study.
5 ConclusionIn conclusion, our study emphasized the critical significance of understanding the severity and clinical presentation of pediatric patients with COVID-19, shedding light on key symptoms and risk factors for severe illness. While fever, cough, and dyspnea remained prevalent symptoms, our findings suggested a relatively low mortality rate among pediatric patients. However, underlying comorbidities, obesity, and gastrointestinal symptoms independently correlated with ICU admission, emphasizing the need for early intervention and monitoring. In addition, our analysis highlighted the role of BMI in disease severity and hospital admission. Moving forward, vaccination efforts and targeted interventions are important in protecting vulnerable pediatric populations and reducing the burden on healthcare systems.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by The Ethics Committee of Mazandaran University of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsAH: Conceptualization, Data curation, Supervision, Writing – review & editing. MRN: Data curation, Writing – review & editing. AHB: Investigation, Writing – original draft, Writing – review & editing. FH: Data curation, Methodology, Writing – original draft, Writing – review & editing. FRM: Formal Analysis, Methodology, Writing – review & editing. MSR: Conceptualization, Data curation, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
FundingThe authors declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsThe authors would like to thank all parents of the children for their cooperation.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1398106/full#supplementary-material
Supplementary Table 1
Clinical characteristics and outcomes of children with COVID-19 in two groups of outpatients and inpatients.
Supplementary Data Sheet 1
Critical preparedness, readiness and response actions for COVID-19: interim guidance published by World Health Organization in 2020.
1. Navaeifar MR, Shahbaznejad L, Sadeghi Lotfabadi A, Rezai MS. COVID-19-Associated multisystem inflammatory syndrome complicated with giant coronary artery aneurysm. Case Rep Pediatr. (2021) 2021:8836403. doi: 10.1155/2021/8836403
PubMed Abstract | Crossref Full Text | Google Scholar
3. Rostami-Maskopaee F, Ladomenou F, Razavi-Amoli SK, Navaeifar MR, Hajialibeig A, Shahbaznejad L, et al. Clinical characteristics and outcomes of the multisystem inflammatory syndrome in children (Mis-C) following COVID-19 infection in Iran: a multicenter study. PLoS One. (2022) 17(9):e0274104. doi: 10.1371/journal.pone.0274104
PubMed Abstract | Crossref Full Text | Google Scholar
4. Shahbaznejad L, Rouhanizadeh H, Navaeifar MR, Hosseinzadeh F, Movahedi FS, Rezai MS. Clinical characteristics and outcomes of COVID-19 in children in northern Iran. Int J Pediatr. (2021) 2021:5558287. doi: 10.1155/2021/5558287
PubMed Abstract | Crossref Full Text | Google Scholar
5. Sedighi I, Fahimzad A, Pak N, Khalili M, Shokrollahi MR, Heydari H, et al. A multicenter retrospective study of clinical features, laboratory characteristics, and outcomes of 166 hospitalized children with coronavirus disease 2019 (COVID-19): a preliminary report from Iranian network for research in viral diseases (inrvd). Pediatr Pulmonol. (2022) 57(2):498–507. doi: 10.1002/ppul.25756
PubMed Abstract | Crossref Full Text | Google Scholar
6. Shahbaznejad L, Navaeifar MR, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. (2020) 20(1):513. doi: 10.1186/s12887-020-02415-z
PubMed Abstract | Crossref Full Text | Google Scholar
7. Zamani R, Zare A, Davoodi SZ, Shati M, Eshaghi H, Faramarzinia A, et al. Early childhood COVID-19: a comparative report of 20,506 cases. Pediatr Infect Dis J. (2023) 42(8):e293–e5. doi: 10.1097/inf.0000000000003955
PubMed Abstract | Crossref Full Text | Google Scholar
8. Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, et al. Susceptibility to sars-cov-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. (2021) 175(2):143–56. doi: 10.1001/jamapediatrics.2020.4573
PubMed Abstract | Crossref Full Text | Google Scholar
9. Avila GA, Daza M, Forero-Motta D, Rozo-Gutierrez N, Osorio J, Walteros DM, et al. Sars-Cov-2 infection in newborn infants: descriptive epidemiological analysis of cases reported to the Colombian national surveillance system during the first pandemic year, March 2020–February 2021. BMJ Paediatr Open. (2023) 7(1):e001781. doi: 10.1136/bmjpo-2022-001781
PubMed Abstract | Crossref Full Text | Google Scholar
10. Rezai MS, Rostami-Maskopaee F, Navaeifar MR, Hajialibeig A, Gooran M, Haghighi Aski B, et al. Multisystem inflammatory syndrome mortality following COVID-19 in Iranian children: a case series and literature review. J Pediatr Rev. (2023) 11(3):231–44. doi: 10.32598/jpr.11.3.1109.1
Crossref Full Text | Google Scholar
11. Mansour Ghanaie R, Boone I, Shamshiri AR, Karimi A, Amirali A, Marhamati N, et al. Seroepidemiological and molecular survey for the detection of sars-cov-2 infection among children in Iran, September 2020 to June 2021: 1-year cross-sectional study. Microorganisms. (2023) 11(7):1672. doi: 10.3390/microorganisms11071672
PubMed Abstract | Crossref Full Text | Google Scholar
12. Berg SK, Palm P, Nielsen SD, Nygaard U, Bundgaard H, Rosenkilde S, et al. Symptoms in the acute phase of sars-cov-2 infection among Danish children aged 0–14 years. IJID Reg. (2023) 7:262–7. doi: 10.1016/j.ijregi.2023.04.012
PubMed Abstract | Crossref Full Text | Google Scholar
13. Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, et al. Risk factors for severe COVID-19 in children. Pediatrics. (2022) 149(1):e2021053418. doi: 10.1542/peds.2021-053418
PubMed Abstract | Crossref Full Text | Google Scholar
15. Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A, et al. Sars-Cov-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. (2021) 21(4):473–81. doi: 10.1016/s1473-3099(20)30858-6
PubMed Abstract | Crossref Full Text | Google Scholar
16. Shamsi F, Karimi M, Nafei Z, Akbarian E. Survival and mortality in hospitalized children with COVID-19: a referral center experience in Yazd, Iran. Can J Infect Dis Med Microbiol. (2023) 2023:5205188. doi: 10.1155/2023/5205188
PubMed Abstract | Crossref Full Text | Google Scholar
17. Mahmoudi S, Pourakbari B, Benvari S, Hosseinpour Sadeghi R, Abdolsalehi MR, Shahbabaie MA, et al. Clinical and laboratory features of sars-cov-2 variants across multiple rounds of pandemic waves in hospitalized children in an Iranian referral hospital. BMC Pediatr. (2023) 23(1):241. doi: 10.1186/s12887-023-04042-w
PubMed Abstract | Crossref Full Text | Google Scholar
18. Fattahi P, Abdi S, Saeedi E, Sirous S, Firuzian F, Mohammadi M, et al. In-Hospital mortality of COVID-19 in Iranian children and youth: a multi-centre retrospective cohort study. J Glob Health. (2022) 12:05048. doi: 10.7189/jogh.12.05048
PubMed Abstract | Crossref Full Text | Google Scholar
19. Mohammadpour M, Hassani SA, Sharifzadeh M, Tahernia L, Mamishi S, Yaghmaie B, et al. COVID-19 pandemic experiences in pediatric intensive care unit: an Iranian referral hospital-based study. Int J Clin Pract. (2022) 2022:1682986. doi: 10.1155/2022/1682986
PubMed Abstract | Crossref Full Text | Google Scholar
20. Mamishi S, Pourakbari B, Mehdizadeh M, Navaeian A, Eshaghi H, Yaghmaei B, et al. Children with sars-cov-2 infection during the novel coronaviral disease (COVID-19) outbreak in Iran: an alarming concern for severity and mortality of the disease. BMC Infect Dis. (2022) 22(1):382. doi: 10.1186/s12879-022-07200-0
PubMed Abstract | Crossref Full Text | Google Scholar
21. Armin S, Fahimzad SA, Rafiei Tabatabaei S, Mansour Ghanaiee R, Marhamati N, Ahmadizadeh SN, et al. COVID-19 mortality in children: a referral center experience from Iran (Mofid Children’s Hospital, Tehran, Iran). Can J Infect Dis Med Microbiol. (2022) 2022:2737719. doi: 10.1155/2022/2737719
PubMed Abstract | Crossref Full Text | Google Scholar
22. Mahmoudi S, Mehdizadeh M, Shervin Badv R, Navaeian A, Pourakbari B, Rostamyan M, et al. The coronavirus disease 2019 (COVID-19) in children: a study in an Iranian children’s referral hospital. Infect Drug Resist. (2020) 13:2649–55. doi: 10.2147/idr.S259064
PubMed Abstract | Crossref Full Text | Google Scholar
23. Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, Navaeifar MR, Enayati AA, Manafi Anari A, et al. COVID-19 infection in Iranian children: a case series of 9 patients. JPR. (2020) 8(2):139–44. doi: 10.32598/jpr.8.2.139
Crossref Full Text | Google Scholar
24. Sarbakhsh P, Jafari N, Salemi S, Akbarnejad R. Predictors of pediatric COVID-19 vaccination: a case-control study in tabriz, Iran. BMC Pediatr. (2023) 23(1):379. doi: 10.1186/s12887-023-04202-y
PubMed Abstract | Crossref Full Text | Google Scholar
25. Charania NA, Kirkpatrick L, Paynter J. Paediatric COVID-19 vaccination coverage and associated factors among migrant and non-migrant children aged 5–11 years in Aotearoa New Zealand: a population-level retrospective cohort study. Aust N Z J Public Health. (2023) 47(5):100086. doi: 10.1016/j.anzjph.2023.100086
PubMed Abstract | Crossref Full Text | Google Scholar
27. Karimi A, Rafiei Tabatabaei S, Rajabnejad M, Pourmoghaddas Z, Rahimi H, Armin S, et al. An algorithmic approach to diagnosis and treatment of coronavirus disease 2019 (COVID-19) in children: Iranian expert’s consensus statement. Arch Pediatr Infect Dis. (2020) 8(2):e102400. doi: 10.5812/pedinfect.102400
Crossref Full Text | Google Scholar
28. Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh M, et al. Clinical and virological features of severe acute respiratory syndrome coronavirus 2 (sars-cov-2) variants of concern: a retrospective cohort study comparing B.1.1.7 (alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. (2022) 75(1):e1128–e36. doi: 10.1093/cid/ciab721
PubMed Abstract | Crossref Full Text | Google Scholar
29. Mustafa ZU, Khan AH, Harun SN, Salman M, Godman B. Antibiotic overprescribing among neonates and children hospitalized with COVID-19 in Pakistan and the implications. Antibiotics (Basel). (2023) 12(4):646. doi: 10.3390/antibiotics12040646
PubMed Abstract | Crossref Full Text | Google Scholar
30. Sefah IA, Sarkodie SA, Pichierri G, Schellack N, Godman B. Assessing the clinical characteristics and management of COVID-19 among pediatric patients in Ghana: findings and implications. Antibiotics (Basel). (2023) 12(2):283. doi: 10.3390/antibiotics12020283
PubMed Abstract | Crossref Full Text | Google Scholar
31. Bhalala US, Gist KM, Tripathi S, Boman K, Kumar VK, Retford L, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med. (2022) 50(1):e40–51. doi: 10.1097/ccm.0000000000005232
PubMed Abstract | Crossref Full Text | Google Scholar
32. Flores-Alanis A, Saldaña-Ahuactzi Z, Parra-Ortega I, López-Ramírez P, Salazar-García M, Alemán-García YP, et al. Clinical characteristics of coronavirus disease (COVID-19) in Mexican children and adolescents. Viruses. (2022) 14(10):2162. doi: 10.3390/v14102162
PubMed Abstract | Crossref Full Text | Google Scholar
33. Buonsenso D, Pazukhina E, Gentili C, Vetrugno L, Morello R, Zona M, et al. The prevalence, characteristics and risk factors of persistent symptoms in non-hospitalized and hospitalized children with sars-cov-2 infection followed-up for up to 12 months: a prospective, cohort study in Rome, Italy. J Clin Med. (2022) 11(22):6772. doi: 10.3390/jcm11226772
PubMed Abstract | Crossref Full Text | Google Scholar
34. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. Br Med J. (2020) 370:m3249. doi: 10.1136/bmj.m3249
PubMed Abstract | Crossref Full Text | Google Scholar
35. Dai YH, Li C, Yuan G, Mo W, Chen J, Huang R, et al. A multicentre study on the clinical characteristics of newborns infected with coronavirus disease 2019 during the omicron wave. Front Pediatr. (2023) 11:1192268. doi: 10.3389/fped.2023.1192268
PubMed Abstract | Crossref Full Text | Google Scholar
36. Ramírez-Cázares AC, Hernández-Ruíz YG, Martínez-Longoria CA, Tamez-Gómez CE, Medina-Macías O, Treviño-Montalvo RG. Clinical characteristics of pediatric patients with confirmed sars-cov-2 infection who followed rigorous measures during two years of the COVID-19 pandemic in a hospital in Mexico. Front Pediatr. (2023) 11:1150738. doi: 10.3389/fped.2023.1150738
PubMed Abstract | Crossref Full Text | Google Scholar
37. Madani S, Shahin S, Yoosefi M, Ahmadi N, Ghasemi E, Koolaji S, et al. Red flags of poor prognosis in pediatric cases of COVID-19: the first 6610 hospitalized children in Iran. BMC Pediatr. (2021) 21(1):563. doi: 10.1186/s12887-021-03030-2
PubMed Abstract | Crossref Full Text | Google Scholar
38. Ibrahim L, Wilson C, Tham D, Corden M, Jani S, Zhang M, et al. The characteristics of sars-cov-2-positive children in Australian hospitals: a predict network study. Med J Aust. (2023) 218(10):460–6. doi: 10.5694
Comments (0)