Anemia is a widely prevalent hematologic condition affecting 24.3% of the world population across all ages (1). It increases with age, reaching a prevalence of 39%–51% among patients aged over 65 years old (2). In patients undergoing percutaneous coronary intervention (PCI), the prevalence of anemia has been reported to be between 10% and 46.4% (2–8). Hemoglobin concentration (Hgb) values serve as the commonly used measurements for diagnosing anemia. The reference Hgb values for defining anemia are largely based on the definition provided by the World Health Organization (WHO) in 1968 (9). The presence of underlying anemia is linked with poor outcomes in cardiac patients including coronary artery disease and congestive heart failure, patients undergoing transcatheter aortic valve replacement, and patients undergoing PCI including in-hospital and long-term adverse events (3, 5, 8, 10–12). In the context of acute coronary syndrome, anemia exacerbates the mismatch between myocardial oxygen supply and demand by reducing oxygen-carrying capacity and simultaneously increasing myocardial consumption through increased cardiac output (13).
In recent years, there has been growing interest in PCI in anatomically complex lesions and higher-risk patients, particularly those with comorbidities and compromised hemodynamic status such as low ejection fraction (14). Impella (Abiomed Inc., Danvers, MA, USA), which is a percutaneous left ventricular assist device (pLVAD), has been increasingly used for mechanical circulatory support during PCI to provide hemodynamic support and enable complete revascularization (15, 16).
Large-bore access procedures such as Impella-supported high-risk PCI (HRPCI) can increase the risk of bleeding. Despite its prevalence and prognostic impact, there are currently no clear guidelines for the management of anemia in PCI patients. As such, anemia has been overlooked in the context of HRPCI. Recognizing this gap, we aimed to investigate the characteristics of anemia patients and assess the association between anemia and adverse outcomes in patients undergoing HRPCI using data from the cVAD PROTECT III study.
Methods Study design, population, and oversightThe PROTECT III study design, rationale, and preliminary results have been previously published (17). In brief, the PROTECT III study is a single-arm, observational study that enrolled 1,237 patients between March 2017 and March 2020. These patients underwent Impella-supported HRPCI across 46 centers in North America. This study is part of the global cVAD registry (NCT04136392), including several separate post-approval studies intended to evaluate the safety and efficacy of Impella mechanical circulatory support use for different cardiovascular conditions (18). The PROTECT III study is an FDA-audited post-approval study on patients undergoing elective or urgent HRPCI procedures (i.e., without cardiogenic shock) and in whom Impella 2.5 or Impella CP was implanted for hemodynamic support during the procedure. The decision to use Impella and the definition of HRPCI were made according to the physician's standard of care. Patients met enrollment criteria once the decision was made to use Impella before or during the index PCI. Bailout pLVAD implantation cases were excluded. The current analysis includes only patients with baseline hemoglobin available. The derivation of the analysis population is described in Figure 1.
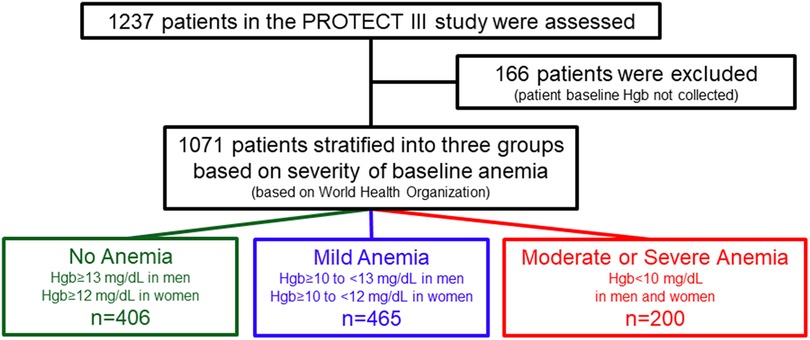
Figure 1 Study flowchart. World Health Organization (2000) has defined mild anemia as hemoglobin 11–11.9 g/dL in women and 11–12.9 g/dL in men; moderate anemia as hemoglobin 8–10.9 g/dL; and severe anemia as hemoglobin <8 g/dL.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the applicable Institutional Review Board or Independent Ethics Committee at each participating site prior to enrollment. Baseline demographics, blood tests, and echocardiography were collected before the index procedure and patients were followed for 90 days. Angiographic data analysis was performed by an independent core lab (Beth Israel Deaconess Medical Center Angiographic Core Laboratory). An independent 12-member steering committee oversaw the conduct of the cVAD study. An independent Clinical Events Committee adjudicated major adverse cardiovascular and cerebrovascular events (MACCE: composite of all-cause mortality, myocardial infarction, stroke/transient ischemic attack (TIA), and repeat revascularization), in addition to adjudicating the relatedness to the study procedure and/or device. The sponsor (Abiomed Inc., Danvers, MA, USA) oversaw study data management and source document verification and provided funding to the Cardiovascular Research Foundation (New York, NY, USA) for statistical analysis. The authors had unrestricted access to the study data and accepted responsibility for the integrity of this report. Artificial intelligence was not utilized at any point throughout the generation of this manuscript.
DefinitionsAnemia was defined according to World Health Organization (WHO) criteria (9). The patients were categorized into three groups: no anemia, characterized by Hgb levels ≥12 mg/dL for women and ≥13 mg/dL for men; mild anemia, with Hgb levels ≥10 to <12 mg/dL in women and ≥10 to <13 mg/dL in men; and moderate or severe anemia, indicated by Hgb levels <10 mg/dL in men and women.
The primary endpoint was MACCE at 30 and 90 days. Secondary endpoints included the following: (1) major bleeding events [Bleeding Academic Research Consortium (BARC) ≥ 3a], (2) duration of hospitalization, (3) major vascular or structural complications requiring surgery or intervention, and (4) 1-year all-cause mortality. Detailed definitions of endpoints have been previously published (19). Prolonged Impella support was defined by any of the following conditions: the site reported that the Impella device was not removed at the end of the procedure, the duration from Impella initiation to PCI completion exceeded 60 min, or additional mechanical support devices were implanted after the Impella was removed.
Statistical analysisThe baseline characteristics were summarized as mean ± standard deviation or median and interquartile range (IQR) for continuous measures and proportions for categorical variables. Between the study groups, categorical variables were summarized as percentages and compared using the chi-squared or Fisher's exact test (e.g., blood transfusion, bleeding, and vascular complications requiring intervention), whereas continuous variables (e.g., duration of hospitalization) were summarized as mean ± standard deviation and median (IQR) and compared using ANOVA and Wilcoxon rank-sum test. For time-to-first event analyses, event rates were estimated by the Kaplan–Meier method and compared using the log-rank test. We tested the normality of continuous variables using the Shapiro–Wilk test. If normality failed per the Shapiro–Wilk test (p < 0.05), then the distributions were compared using the Wilcoxon rank-sum test. The Cox proportional hazard model is applied to compare anemia groups and their association with 30-day and 90-day MACCE rates after adjustment for sex, age, left ventricular ejection fraction (LVEF), and eGFR.
Multivariable logistic regression was done to compare anemia groups and bleeding events after adjustment for the same confounders as in the Cox regression model. Additional logistic regression analysis was performed to compare the need for blood transfusion after adjustment for age, sex, anticoagulation treatment, dialysis, peripheral artery disease, and prolonged Impella support between anemia groups. The variables included in the multivariable Cox proportional hazard regression for 30-day and 90-day MACCE, and those included in multivariable logistic regression models were selected based on prior evidence in the literature and their effect in univariable analysis.
An additional sensitivity analysis excluding patients on dialysis was performed for the primary endpoint and 1-year mortality using Kaplan–Meier time-to-event estimation. To compare between anemia groups MACCE rates at 30- and 90-day Cox proportional hazards regression model excluding patients on dialysis was completed and adjusted to the same confounders as in the entire study population. Multiple imputation was used to account for missing data for covariates in the multivariable Cox and logistic regression analyses. For this analysis, we used the Markov chain Monte Carlo method. All p-values are two-tailed, and p < 0.05 was considered significant for all analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Data transparency and opennessBecause of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the study sponsor (Abiomed) at aalmedhychy@abiomed.com.
Results Baseline characteristicsFrom March 2017 to March 2020, 1,237 consecutive patients were enrolled in the PROTECT III study at 46 sites in North America, of whom 1,071 had baseline Hgb available (Figure 1). At baseline, 37.9% of patients (406) had no anemia, 43.4% (465) had mild anemia, and 18.7% (200) had moderate to severe anemia. Baseline patient characteristics are presented in Table 1. Patients with anemia, compared to those without, were characterized by older age and a higher burden of comorbidities including chronic kidney disease, peripheral vascular diseases, and diabetes mellitus. In addition, the proportion of female patients was higher in the two anemia groups than in the no anemia group, although males were predominant in all groups. There were no significant differences in the rates of prior PCI or previous coronary artery bypass grafting between the three groups. Patients with anemia were more likely to have lower left ventricular ejection fraction (LVEF) (ANOVA p-value = 0.002), but there were no significant differences in right ventricle function or valvular pathologies.
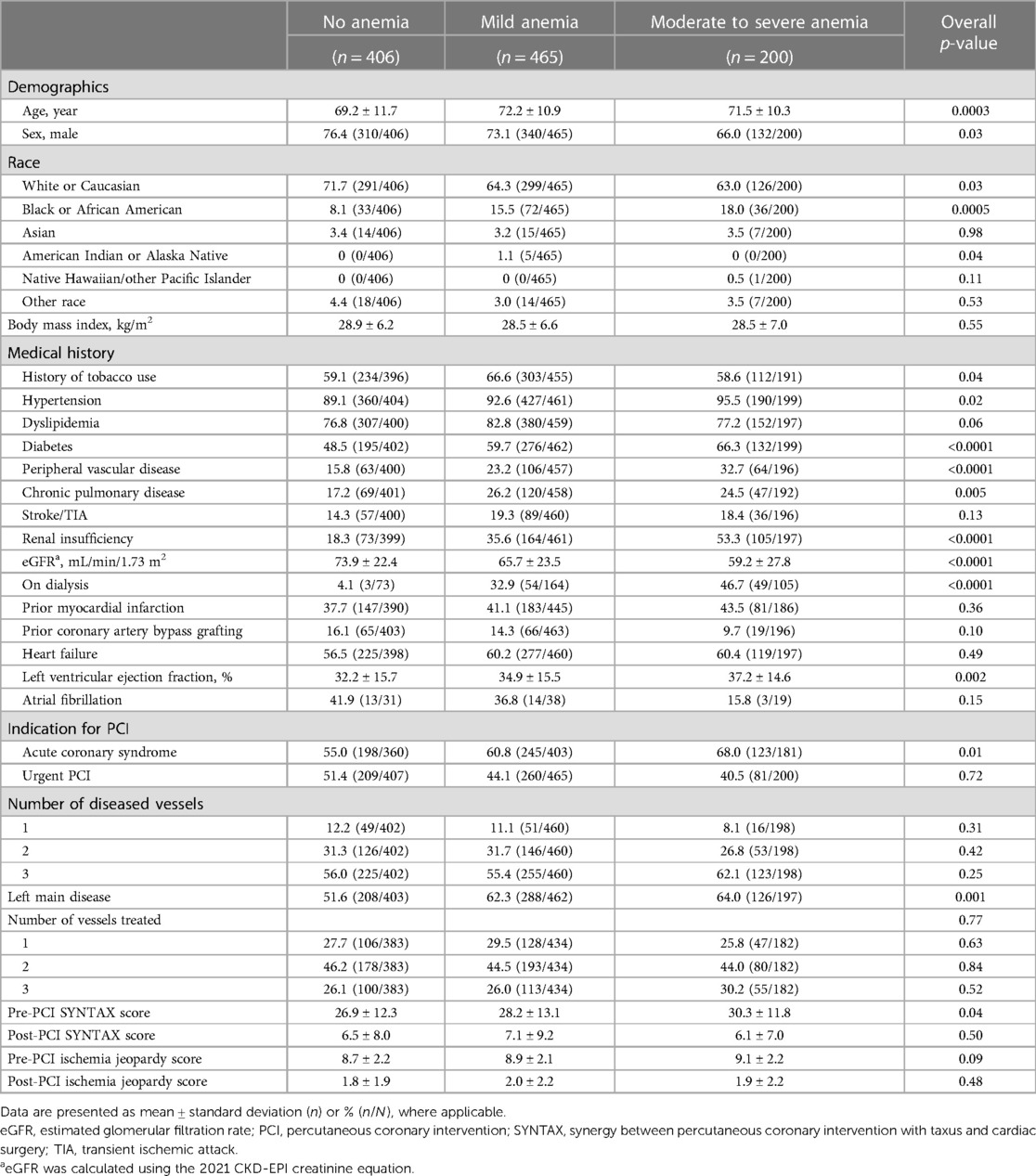
Table 1 Baseline demographics and procedural characteristics according to anemia severity.
Admission and procedural characteristicsPatients with moderate to severe anemia had higher rates of acute coronary syndrome as the primary reason for admission compared to patients with mild or no anemia (68.0% vs. 60.8% vs. 55.0% respectively, chi-squared p-value = 0.01).
Both patients with moderate to severe anemia and those with mild anemia had a significantly higher incidence of left main (LM) disease compared to patients with no anemia (64.0% vs. 62.3% vs. 51.6% respectively, chi-squared p-value = 0.001). Patients with baseline anemia also had higher pre-PCI SYNTAX scores, longer duration of PCI procedure, and longer hospitalization time with greater need for blood transfusion compared to patients with no anemia (Table 2). However, there was no significant difference in post-PCI SYNTAX score or Impella support duration between groups.
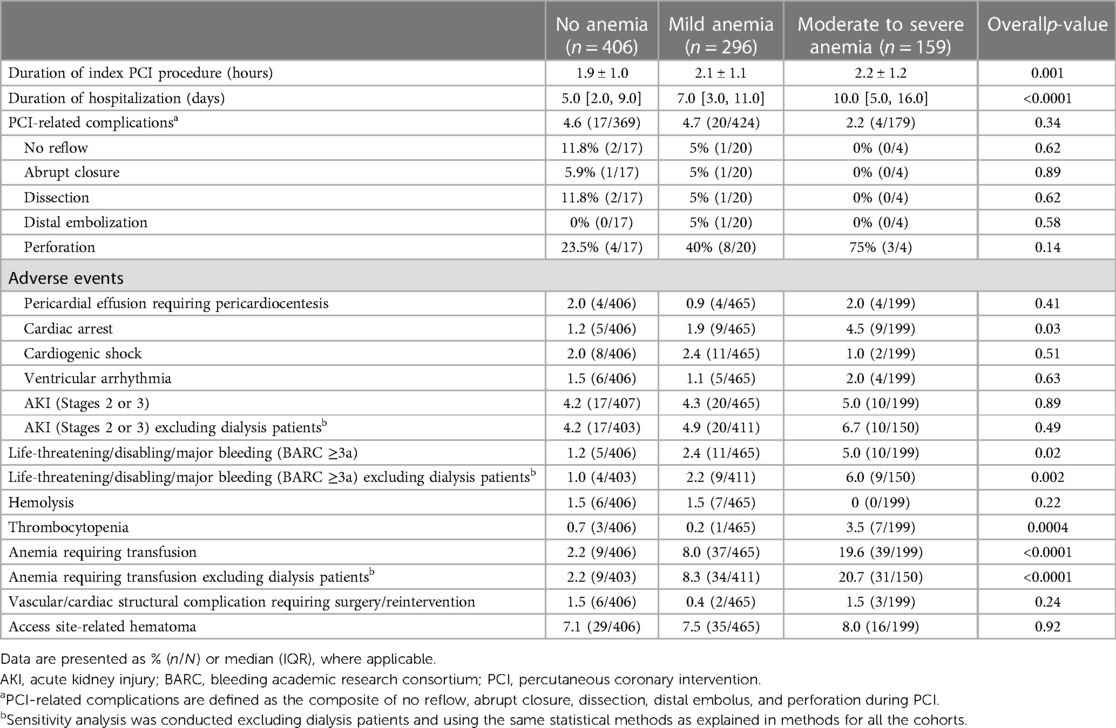
Table 2 Immediate PCI-related complications and in-hospital adverse events according to anemia severity.
Major adverse cardiovascular and cerebrovascular eventsUnivariate analysis was performed for 30-day and 90-day MACCE. At 30 days, patients with baseline moderate to severe anemia had significantly higher MACCE rates compared to patients with mild anemia or no anemia (12.3% vs. 9.8% vs. 5.4% respectively, overall log-rank p-value = 0.02). This difference persisted at 90 days with MACCE rates among the moderate to severe anemia group being 18.7% compared to 14.6% in patients with mild anemia and 8.4% in patients with no anemia (overall log-rank p-value = 0.004; Figure 2, Table 3). Multivariable analysis was then performed adjusting for age, sex, LVEF, and eGFR. The findings from multivariable analysis remained consistent showing that both mild and moderate to severe anemia were associated with higher MACCE rates at 30 and 90 days (Figure 3). A sensitivity analysis excluding patients with dialysis was conducted. Of the 1,071 patients with baseline hemoglobin measurements, 106 patients were reported to be on dialysis. Among them, 46.7% (49 patients) had moderate to severe anemia. In this analysis unadjusted MACCE rates remained higher among patients with anemia at 30 days (log-rank p = 0.046) and 90 days (log-rank p = 0.02) (Supplementary Table S1 and Figure S1), as well as all-cause mortality at 1 year (log-rank p = 0.002) (Supplementary Table S1 and Figure S2). However, after adjusting for confounders (age, sex, left ventricular ejection fraction, eGFR, and anemia group) MACCE rates are significantly higher among the moderate to severe anemia group at 90 days and non-significant at 30 days, moreover mild anemia was not a significant predictor of MACCE outcomes in this adjusted model (Supplementary Figure S3).
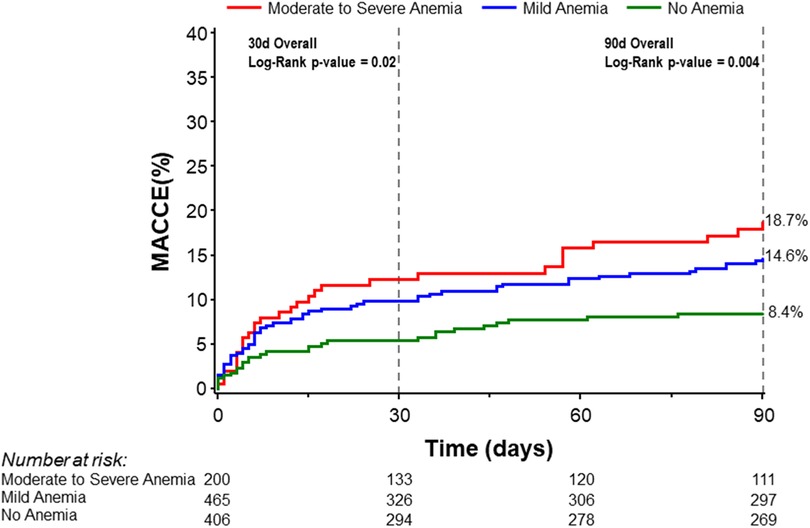
Figure 2 Kaplan–Meier curves for 30- and 90-day MACCE stratified by severity of anemia. MACCE denotes major adverse cardiovascular and cerebrovascular events.

Table 3 Ninety-day major adverse cardiac and cerebrovascular events at 90 days and mortality at 1 year according to anemia severity.
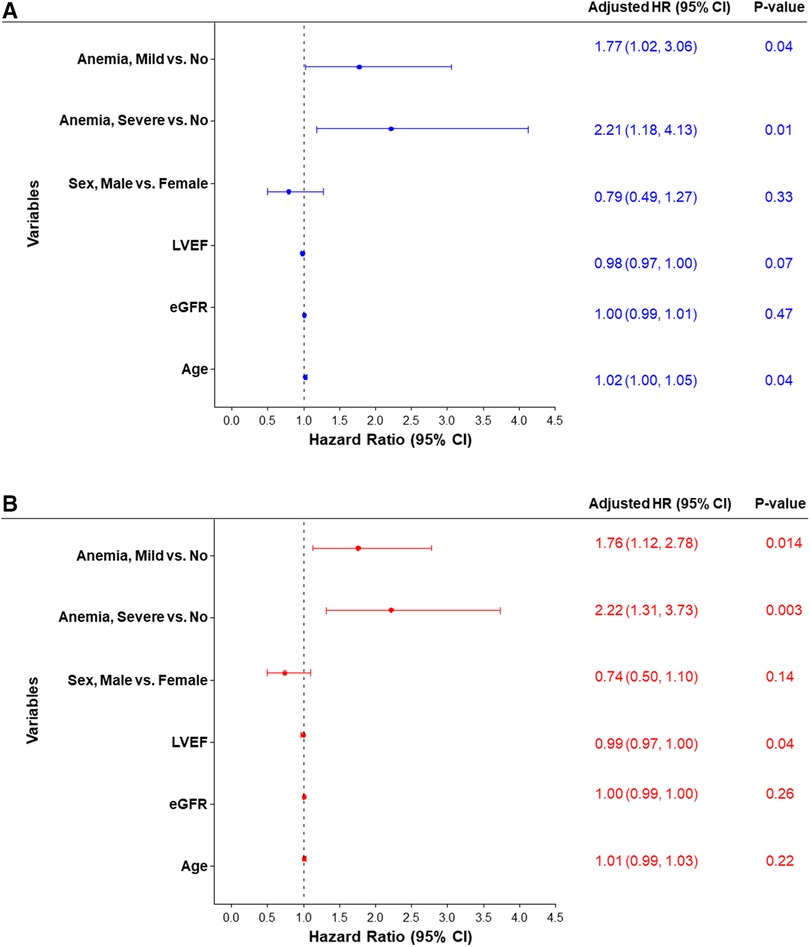
Figure 3 Forest plot of the adjusted hazard ratio for MACCE rates estimated by cox proportional hazards regression models at (A) 30 days and (B) 90 days. eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction.
Secondary endpointsA greater proportion of patients with moderate to severe anemia and mild anemia had major bleeding incidents (BARC ≥ 3a) compared to patients with no anemia (5.0% vs. 2.4% vs. 1.2%, Chi-square p-value = 0.02; Table 2). Moreover, blood transfusion was significantly higher among anemic patients (Chi-square p-value <0.0001). This difference remained statistically significant after excluding patients on dialysis (Chi-square p-value <0.0001; Table 2). Patients with moderate to severe anemia were hospitalized longer compared to mild anemia or no anemia groups [10.0 (5.0, 16.0) days vs. 7.0 (3.0, 11.0) days vs. 5.0 (2.0, 9.0), Wilcoxon rank-sum p-value <0.0001; Table 2]. There were no significant differences in major vascular complications requiring intervention. Notably, one-year mortality was significantly higher in the moderate to severe anemia group than in the mild and no anemia group (31.8%, 23.2%, and 15.5%, respectively; log-rank p-value = 0.0002; Table 3, Figure 4).
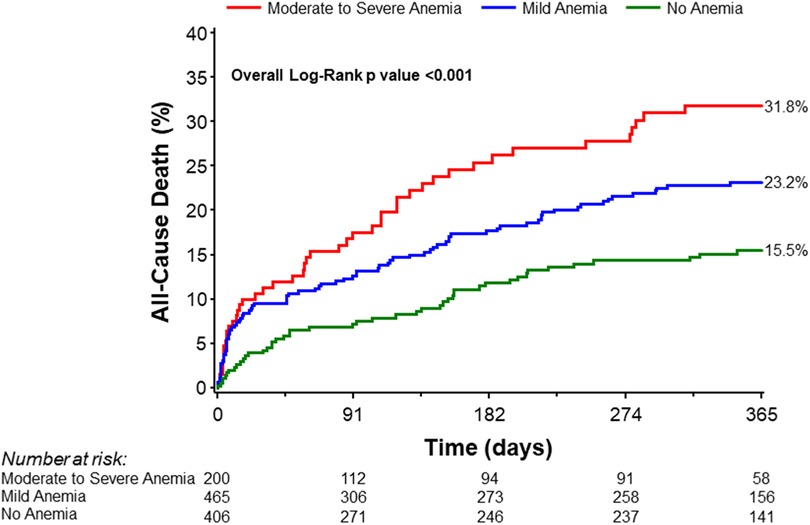
Figure 4 Kaplan–Meier curves for all-cause mortality at 1 year stratified by severity of anemia.
Multiple logistic regression models adjusting for age, sex, LVEF, eGFR, and anemia group indicated that moderate to severe anemia was independently associated with major bleeding [Adjusted OR (95% CI): 2.53 (1.37, 4.68), p-value = 0.003] (Supplementary Table S2). Further analysis on predictors of blood transfusion after adjusting for multiple covariates (age, anticoagulation treatment, dialysis, peripheral artery disease, sex, prolonged Impella support, and anemia group) revealed that moderate or severe anemia, peripheral vascular disease, and prolonged Impella support were independently associated with blood transfusion requirements (Figure 5).
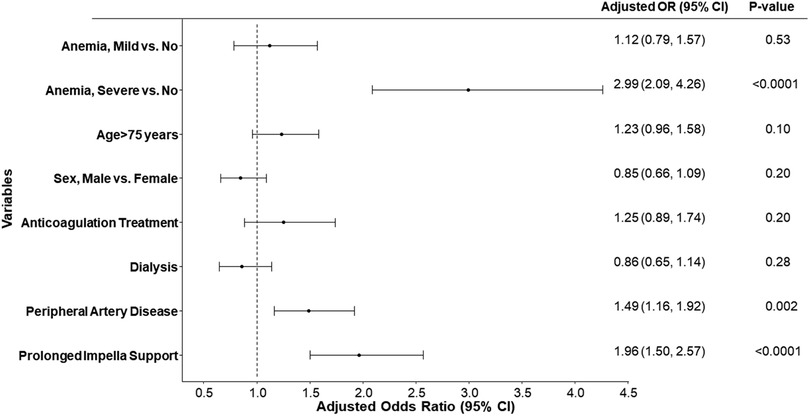
Figure 5 Forest plot of the adjusted odds ratio for blood transfusion for different variables. Odds ratios (OR) and 95% confidence intervals (95% CI) are estimated by multiple logistic regression models. Prolonged support Impella is defined as any Impella support beyond the index procedure.
DiscussionIn this study assessing outcomes in pLVAD-supported HRPCI, we found that baseline anemia is common and associated with MACCE, bleeding events, and death in this population.
Several previous studies have indicated an association between baseline anemia and adverse outcomes in contemporary PCI with a twofold increased risk of mortality and MACE events, as well as an elevated risk of bleeding with an incremental decrease in hemoglobin level (20). Similarly, a report from the Myocardial Infarction Data Acquisition System suggests a higher 1-year mortality among patients with vs. without anemia (21). However, other studies found no increase in risk after adjustment for age, comorbidity burden, and procedural demographics (22, 23). The association between baseline anemia and outcomes in Impella-supported HRPCI has not previously been examined. Based on our results, anemia should be considered in the risk stratification of patients intended for HRPCI.
There is an ongoing debate about whether anemia is simply a marker of sicker more medically complex patients or an independent predictor of adverse clinical outcomes. Within our cohort, anemia was more prevalent in patients with underlying comorbidities. However, this increased risk persisted in the moderate to severe anemia group after adjustment for potential confounding factors (age, renal function, LVEF, and sex). Given the high prevalence of anemia among dialysis patients (24) and that dialysis is independently associated with increased risk for adverse cardiovascular events and procedure complications (25–27), we conducted a sensitivity analysis excluding dialysis patients. The result of this analysis showed that only moderate to severe anemia was independently associated with higher MACCE rates at 90 days. This suggests that, for this patient group, lower hemoglobin cutoffs should be considered in risk assessments compared to non-dialysis patients. These findings suggest that this association between anemia and MACCE rates might be affected by other confounders and is more evident in patients with moderate or severe anemia rather than mild anemia.
Several hypotheses may account for the observed association between anemia and adverse PCI outcomes. An imbalance between myocardial oxygen supply and demand may be exacerbated by anemia due to the combination of reduced oxygen-carrying capacity and increased myocardial oxygen consumption through increased cardiac output associated with severe anemia and obstructive coronary disease. Additionally, there is a link between nitric oxide and Hgb, further influencing vascular function and causing impaired vascular relaxation and healing, which is exacerbated by an inflammatory response that is inversely correlated with anemia in acute coronary syndrome patients (13).
We also investigated the impact of anemia on major bleeding events (BARC ≥ 3a) and blood transfusions. Results of a logistic regression model for blood transfusion showed that moderate to severe anemia prolonged Impella support and peripheral vascular disease were independent factors associated with blood transfusion. This suggests that patients with baseline anemia have more hemolysis during Impella support and impaired hemostasis worsening their existing baseline anemia and necessitating blood transfusion. These findings raise the question of whether pre-procedure blood transfusion should be considered in certain cases along with a more meticulous access and closure technique planning to minimize procedural complications. Periprocedural anticoagulation strategy and choice of antiplatelets should be carefully assessed weighing the risks vs. benefits. Additionally, given that prolonged Impella support was also associated with blood transfusion, it may suggest that removal of Impella should be done as promptly as possible once the patient is stabilized and no longer in need of hemodynamic support.
Current clinical guidelines lack recommendations for the concurrent management of anemia in patients undergoing PCI or large-bore procedures such as Impella-supported HRPCI, other than advising measures to minimize bleeding risks and utilizing risk scores to guide dual antiplatelet therapy. Regarding blood transfusion treatment in general, the recent randomized MINT study, comparing restrictive or liberal transfusion strategies in myocardial infarction and anemia, found that the liberal transfusion strategy did not significantly reduce the risk of recurrent myocardial infarction or death at 30 days (28). It should be noted that this trial also included patients who did not have PCI. The lack of specific guidelines and integration of formal bleeding risk algorithms into routine care renders the management of patients with anemia who require high-risk PCI difficult.
Study limitationsOur study has several limitations. First, this is a post hoc analysis derived from a single-arm observational study and thus causality cannot be determined. Additionally, the management and treatment of anemia were at the discretion of the operators and therefore may have varied both between individual operators and across medical centers.
In conclusion, our study shows that moderate to severe anemia is an independent predictor of MACCE and bleeding after Impella-supported complex PCI, portending a worse prognosis. The extent to which these associations between anemia and adverse events after complex PCI can be mitigated by optimized anemia management and tailored interventions remains to be established.
Data availability statementThe datasets presented in this article are not readily available because of the sensitive nature of the data collected for this study. Requests to access the datasets from qualified researchers trained in human subject confidentiality protocols should be directed to the study Sponsor (Abiomed) at aalmedhychy@abiomed.com.
Ethics statementThe studies involving humans were conducted in accordance with the Declaration of Helsinki and were approved by the applicable Institutional Review Board or Independent Ethics Committee at each participating site prior to enrollment. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsBF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. BR: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. DZ: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. ASB: Investigation, Writing – original draft, Writing – review & editing. MBB: Investigation, Writing – original draft, Writing – review & editing. JBT: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. RAGP: Investigation, Writing – original draft, Writing – review & editing. MJS: Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. AA-M: Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. WBB: Investigation, Writing – original draft, Writing – review & editing. CLG: Investigation, Writing – original draft, Writing – review & editing. WWO: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
FundingThe authors declare that financial support was received for the research, authorship, and/or publication of this article.
The PROTECT III study, as part of the Global cVAD study, was sponsored by Abiomed Inc. (Danvers, MA, USA).
AcknowledgmentsThe co-authors would like to thank Maria C. Alu, MS (Clinical Trials Center, Cardiovascular Research Foundation, New York, NY, USA) for editing the manuscript.
Conflict of interestBR reports consultant fees from Pfizer and Boehringer Ingelheim. ASB reports consulting and speaker fees from Abiomed, Shockwave Medical, and Cardiovascular Systems, Inc. MBB discloses consultant/speaker fees from Abiomed, Boston Scientific, Chiesi, Saranas, and Zoll. RAGP reports consultant/speaker fees from Abiomed; speaker honoraria Boston Scientific. WBB reports consultant fees from Abbott, Medtronic, Abiomed, and Boston Scientific. CLG reports participation on the advisory boards for Philips and Abiomed. WWO reports grant/research support from St. Jude Medical, Edwards Lifesciences, and BioMed; consulting fees/honoraria from Medtronic and Abiomed; and major stock shareholder/equity in Synecor, Accumed, Neovasc, Tendyne, and Mitralign.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1429900/full#supplementary-material
AbbreviationsBARC, Bleeding Academic Research Consortium; Hgb, hemoglobin; HRPCI, high-risk percutaneous coronary intervention; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiovascular and cerebrovascular event; PCI, percutaneous coronary intervention; pLVAD, percutaneous left ventricular assist device; WHO, World Health Organization.
References1. Collaborators GBDA. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the Global Burden of Disease Study 2021. Lancet Haematol. (2023) 10:e713–34. doi: 10.1016/S2352-3026(23)00160-6
PubMed Abstract | Crossref Full Text | Google Scholar
2. AÏdoud A, Gana W, Poitau F, Debacq C, Leroy V, Nkodo JA, et al. High prevalence of geriatric conditions among older adults with cardiovascular disease. J Am Heart Assoc. (2023) 12(2):e026850. doi: 10.1161/JAHA.122.026850
PubMed Abstract | Crossref Full Text | Google Scholar
3. Anker SD, Voors A, Okonko D, Clark AL, James MK, von Haehling S, et al. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: data from the OPTIMAAL trial. Eur Heart J. (2009) 30(11):1331–9. doi: 10.1093/eurheartj/ehp116
PubMed Abstract | Crossref Full Text | Google Scholar
4. Faggioni M, Baber U, Sartori S, Chandrasekhar J, Cohen DJ, Henry TD, et al. Influence of baseline anemia on dual antiplatelet therapy cessation and risk of adverse events after percutaneous coronary intervention. Circ Cardiovasc Interv. (2019) 12(4):e007133. doi: 10.1161/CIRCINTERVENTIONS.118.007133
PubMed Abstract | Crossref Full Text | Google Scholar
5. Tsujita K, Nikolsky E, Lansky AJ, Dangas G, Fahy M, Brodie BR, et al. Impact of anemia on clinical outcomes of patients with ST-segment elevation myocardial infarction in relation to gender and adjunctive antithrombotic therapy (from the HORIZONS-AMI trial). Am J Cardiol. (2010) 105(10):1385–94. doi: 10.1016/j.amjcard.2010.01.001
PubMed Abstract | Crossref Full Text | Google Scholar
6. Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. (2005) 111(16):2042–9. doi: 10.1161/01.CIR.0000162477.70955.5F
PubMed Abstract | Crossref Full Text | Google Scholar
7. Nikolsky E, Aymong ED, Halkin A, Grines CL, Cox DA, Garcia E, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. (2004) 44(3):547–53. doi: 10.1016/j.jacc.2004.03.080
PubMed Abstract | Crossref Full Text | Google Scholar
8. Guedeney P, Sorrentino S, Claessen B, Mehran R. The link between anemia and adverse outcomes in patients with acute coronary syndrome. Expert Rev Cardiovasc Ther. (2019) 17:151–9. doi: 10.1080/14779072.2019.1575729
PubMed Abstract | Crossref Full Text | Google Scholar
9. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. (1968) 405:5–37. PMID: 4975372
PubMed Abstract | Google Scholar
10. Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. (2006) 113:2454–61. doi: 10.1161/CIRCULATIONAHA.105.583666
PubMed Abstract | Crossref Full Text | Google Scholar
11. Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. (2005) 165(19):2237–44. doi: 10.1001/archinte.165.19.2237
PubMed Abstract | Crossref Full Text | Google Scholar
12. Razuk V, Camaj A, Cao D, Nicolas J, Hengstenberg C, Sartori S, et al. Impact of anemia on short-term outcomes after TAVR: a subgroup analysis from the BRAVO-3 randomized trial. Catheter Cardiovasc Interv. (2021) 98(6):E870–80. doi: 10.1002/ccd.29753
PubMed Abstract | Crossref Full Text | Google Scholar
14. Kirtane AJ, Doshi D, Leon MB, Lasala JM, Ohman EM, O'Neill WW, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. (2016) 134(5):422–31. doi: 10.1161/CIRCULATIONAHA.116.022061
PubMed Abstract | Crossref Full Text | Google Scholar
15. Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. (2020) 141(4):273–84. doi: 10.1161/CIRCULATIONAHA.119.044007
PubMed Abstract | Crossref Full Text | Google Scholar
16. Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ, et al. Hospital variation in the utilization of short-term nondurable mechanical circulatory support in myocardial infarction complicated by cardiogenic shock. Circ Cardiovasc Interv. (2019) 12(1):e007270. doi: 10.1161/CIRCINTERVENTIONS.118.007270
PubMed Abstract | Crossref Full Text | Google Scholar
17. O'Neill WW, Anderson M, Burkhoff D, Grines CL, Kapur NK, Lansky AJ, et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am Heart J. (2022) 248:139–49. doi: 10.1016/j.ahj.2022.02.006
PubMed Abstract | Crossref Full Text | Google Scholar
18. Vetrovec GW, Anderson M, Schreiber T, Popma J, Lombardi W, Maini B, et al. The cVAD registry for percutaneous temporary hemodynamic support: a prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am Heart J. (2018) 199:115–21. doi: 10.1016/j.ahj.2017.09.007
PubMed Abstract | Crossref Full Text | Google Scholar
19. Shah T, Abu-Much A, Batchelor WB, Grines CL, Baron SJ, Zhou Z, et al. Sex differences in pLVAD-assisted high-risk percutaneous coronary intervention: insights from the PROTECT III study. JACC Cardiovasc Interv. (2023) 16(14):1721–9. doi: 10.1016/j.jcin.2023.04.036
PubMed Abstract | Crossref Full Text | Google Scholar
20. Kwok CS, Tiong D, Pradhan A, Andreou AY, Nolan J, Bertrand OF, et al. Meta-analysis of the prognostic impact of anemia in patients undergoing percutaneous coronary intervention. Am J Cardiol. (2016) 118:610–20. doi: 10.1016/j.amjcard.2016.05.059
PubMed Abstract | Crossref Full Text | Google Scholar
21. Al Falluji N, Lawrence-Nelson J, Kostis JB, Lacy CR, Ranjan R, Wilson Ac. Effect of anemia on 1-year mortality in patients with acute myocardial infarction. Am Heart J. (2002) 144(4):636–41. doi: 10.1016/S0002-8703(02)00134-5
PubMed Abstract | Crossref Full Text | Google Scholar
22. Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, et al. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interv. (2012) 5(2):202–10. doi: 10.1161/CIRCINTERVENTIONS.111.965749
PubMed Abstract | Crossref Full Text | Google Scholar
23. Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. (2009) 2(3):222–9. doi: 10.1161/CIRCINTERVENTIONS.108.846741
PubMed Abstract | Crossref Full Text | Google Scholar
25. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
PubMed Abstract | Crossref Full Text | Google Scholar
26. Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: a high-risk combination. Am J Kidney Dis. (2005) 45:223–32. doi: 10.1053/j.ajkd.2004.09.022
PubMed Abstract | Crossref Full Text | Google Scholar
27. Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. (2008) 2
Comments (0)