PURPOSE We conducted a meta-analysis to evaluate clinically meaningful benefits and harms of monoclonal antibodies targeting amyloid in patients with Alzheimer dementia.
METHODS We searched PubMed, Cochrane CENTRAL, and 5 trial registries, as well as the reference lists of identified studies. We included randomized controlled trials comparing a monoclonal antibody with placebo at a dose consistent with that used in phase 3 trials or for Food and Drug Administration approval. Studies had to report at least 1 clinically relevant benefit or harm. Data were extracted independently by at least 2 researchers for random effects meta-analysis. Changes in cognitive and functional scales were compared between groups, and each difference was assessed to determine if it met the minimal clinically important difference (MCID).
RESULTS We identified 19 publications with 23,202 total participants that evaluated 8 anti-amyloid antibodies. There were small improvements over placebo in the Alzheimer’s Disease Assessment Scale (ADAS)-Cog-11 to -14 score (standardized mean difference = −0.07; 95% CI, −0.10 to −0.04), Mini Mental State Examination score (0.32 points; 95% CI, 0.13 to 0.50), and Clinical Dementia Rating-Sum of Boxes scale score (mean difference =−0.18 points; 95% CI, −0.34 to −0.03), and the combined functional scores (standardized mean difference = 0.09; 95% CI, 0.05 to 0.13). None of the changes, including those for lecanemab, aducanumab, and donanemab, exceeded the MCID. Harms included significantly increased risks of amyloid-related imaging abnormalities (ARIA)-edema (relative risk [RR] = 10.29; number needed to harm [NNH] = 9), ARIA-hemorrhage (RR = 1.74; NNH = 13), and symptomatic ARIA-edema (RR = 24.3; NNH = 86).
CONCLUSIONS Although monoclonal antibodies targeting amyloid provide small benefits on cognitive and functional scales in patients with Alzheimer dementia, these improvements are far below the MCID for each outcome and are accompanied by clinically meaningful harms.
Key words:INTRODUCTIONThe hypothesis that amyloid deposition is part of the causal pathway in the pathogenesis of Alzheimer dementia has led to the development of monoclonal antibodies to reduce this deposition.1,2 In fact, the primary justification for approval of these drugs by the Food and Drug Administration (FDA) is reduced amyloid deposition in the brain.3,4 Their approval despite their failure to provide a clinically significant improvement in cognitive and functional outcomes has resulted in substantial controversy,3-6 including charges of research misconduct in some of the original studies.7
Surrogate outcomes often do not correspond to improvements in patient-oriented outcomes such as reduced mortality or morbidity. For example, 3 large trials in patients with diabetes found that a lower glycated hemoglobin target of 6.5% either did not reduce or increased mortality compared with standard targets of 7.0% to 8.0%.8-10 A clear focus on patient-oriented benefits and harms is thus central to evidence-based practice.11,12 A recent systematic review concluded that patients with dementia most value quality of life, self-efficacy, and avoidance of depression.13
Previous systematic reviews have evaluated the efficacy and harms of monoclonal antibodies targeting amyloid.14-16 These reviews were, however, unable to include several recent studies that were critical to drug approval. The reviews also in some cases included phase 1 and 2 trials that used different doses from those used in later trials, and did not interpret the findings in the context of the minimal clinically important difference (MCID) for each outcome.
Recently, the monoclonal antibodies lecanemab and aducanumab were studied in large randomized controlled trials that found substantial reductions in amyloid deposition but only modest improvements in cognition and function.1,17,18 Significant harms were observed, including symptomatic amyloid-related imaging abnormalities of edema (ARIA-E) and hemorrhage (ARIA-H). We set out to perform a meta-analysis of all randomized controlled trials comparing an anti-amyloid monoclonal antibody with placebo. Our sole focus was on patient-oriented outcomes, which we defined as improved cognition and/or function attaining at least the MCID for each scale, and potentially serious harms such as cerebral edema, hemorrhage, serious adverse events, and mortality.
METHODSOur protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) as protocol CRD42023392698. The review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement for reporting systematic reviews (Supplemental Appendix 1).
Inclusion and Exclusion CriteriaWe included randomized controlled trials that compared a monoclonal antibody intended to decrease the amount of brain amyloid with placebo. All trials had to enroll adults with cognitive impairment, Alzheimer disease of any severity, or high risk for Alzheimer disease, and had to report at least 1 patient-oriented benefit or harm after a minimum of 1 year. There were no limits by year or language. We excluded trials reporting the results of only a single infusion and phase 1 trials, as well as trials or trial arms using doses lower than those used in phase 3 trials or ultimately approved by the FDA.
Data Abstraction and Quality AssessmentTitles and abstracts were reviewed in parallel by 2 researchers, at least 1 of whom was a physician. Any study identified as potentially relevant by at least 1 researcher was selected for full text review. Full text review was performed in parallel, again with at least 1 physician researcher for each study, to identify studies meeting our inclusion and exclusion criteria.
Abstraction of study characteristics, assessment of study quality, and abstraction of outcome data were done in parallel by 2 researchers, 1 of whom was a physician. The second physician helped resolve any discrepancies between the first 2 reviewers. The quality assessment used the Cochrane Risk of Bias Tool.19 Details regarding the data preparation for 2 studies requiring slight modifications are given in Supplemental Appendix 2.
AnalysisWe performed a random effects meta-analysis of each outcome using the metan procedure in Stata version 17 (StataCorp LLC). For dichotomous outcomes, we calculated relative risks (RRs), 95% CIs, and where relevant, the number needed to treat (NNT) or number needed to harm (NNH). For continuous outcomes, we calculated the mean difference (MD) or when combining similar continuous scales but with different ranges (eg, the Alzheimer’s Disease Assessment Scale Cognitive Subscale-11 items through -14 items [ADAS-Cog-11 through ADAS-Cog-14]), we used the Cohen procedure for calculating summary estimates of the standardized mean difference (SMD).
Forest plots were created for each outcome. Heterogeneity was measured using the I2 statistic.20 Publication bias was assessed using funnel plots for key outcomes using all available studies.
MCID DeterminationThe MCID is the smallest change in a scale measuring cognition or function that is noticeable by the patient or their caregiver. Jaeschke and colleagues21 estimate that for a 7-point scale, a change of 0.5 points (7% of the range) represents the MCID, with changes of 11.5% to 13.7% representing a moderate effect and 12.3% to 21.0% representing a large change. We determined from the literature the MCID for each scale used in 2 or more studies. Where there was no published MCID, we used 7% of the full range of the scale, for example, a change of at least 1.4 points on a 20-point scale. For SMDs, previous research has concluded that a standardized difference of 0.5 should be considered the MCID.22,23 The range and MCID for each scale are shown in Table 1.
Table 1.Cognitive Scoring Tools and Their MCIDs
RESULTSSearch ResultsThe results of our literature search are summarized in Supplemental Figure 1 and Supplemental Table 1. We identified 87 studies from PubMed and 71 from other sources, of which 16 were duplicates. A total of 142 records were screened and 41 underwent full text review. We excluded some studies that initially appeared promising but used subtherapeutic doses,34 studied an anti-Tau antibody,35 or were phase 1 studies.36-40 Two studies reported data regarding ARIA-E outcomes for the same pair of phase 3 trials29,41; we used the information from the more detailed report.41 Just before submitting the revised manuscript we added 2 recently published studies that met our criteria.42,43 Ultimately, we included 19 studies with 23,202 participants evaluating 8 anti-amyloid antibodies.
Study CharacteristicsCharacteristics of the 19 included studies are summarized in Table 2. All studies were industry-funded, placebo-controlled randomized trials. Most were 18 to 19 months in duration, and enrolled patients with mild cognitive impairment or with mild or moderate Alzheimer disease.
Table 2.Characteristics of the 19 Included Studies
Risk of Bias AssessmentThe risk of bias assessment for each study is summarized in Table 3. Twelve studies were at high risk for bias because of a lack of complete outcome data (>10% missing). Four studies were at unclear risk for bias because of uncertainty about allocation concealment. The remaining 3 studies were at low risk for bias.
Table 3.Study Quality Assessment Using the Cochrane Risk of Bias Tool19
Potential BenefitsForest plots of the summary estimates of the SMD for the combined ADAS-Cog-11 through -14 cognitive scores are shown in Figure 1 (individual forest plots for each scale are shown in Supplemental Figures 2-5). The overall improvement with anti-amyloid antibodies over placebo was small (SMD = −0.07; 95% CI, −0.10 to −0.04). Statistically significant improvements in one of these cognitive scores were seen for solanezumab (SMD = 0.07; 95% CI, −0.12 to −0.02), aducanumab (SMD = −0.11; 95% CI, −0.19 to −0.02), and lecanemab (SMD = −0.11; 95% CI, −0.19 to −0.02). For the 2 FDA-approved antibodies, the MD for lecanemab (−1.8 points; 95% CI, −3.1 to −0.52 points) did not exceed the MCID of 4 to 5 points for the ADAS-Cog-14 and the MD for aducanumab (−0.98 points; 95% CI, −1.77 to −0.18 points) did not exceed the MCID for the ADAS-Cog-13 of 3.75 points.24 For donanemab, which is pending FDA approval, the unstandardized MD for change in the ADAS-Cog-13 score was −1.41 points (95% CI, −2.11 to −0.70).
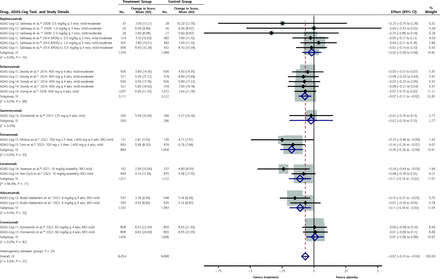
 Figure 1.
Figure 1. Forest plot for the standardized mean differences in ADAS-Cog-11 through ADAS-Cog-14 scores.
ADAS-Cog-11 = Alzheimer’s Disease Assessment Scale–Cognitive Subscale-11 items; ADAS-Cog-12 = Alzheimer’s Disease Assessment Scale–Cognitive Subscale-12 items; ADAS-Cog-13 = Alzheimer’s Disease Assessment Scale–Cognitive Subscale-13 items; ADAS-Cog-14 = Alzheimer’s Disease Assessment Scale–Cognitive Subscale-14 items; IV =interstudy variance; MCI = mild cognitive impairment.
Results for the Mini Mental State Examination (MMSE) cognitive score are shown in Figure 2. The score was improved relative to placebo for all of the anti-amyloid antibodies combined by 0.32 points (95% CI, 0.13 to 0.50). The MMSE was improved by a statistically significant extent but not by a clinically significant extent for solanezumab (MD = 0.53 points; 95% CI, 0.15 to 0.80). For the FDA-approved drugs, the MMSE was not significantly better with aducanumab (MD = 0.25 points; 95% CI, −0.44 to 0.93), while there was a statistically significant benefit for donanemab (MD = 0.49 points; 95% CI, 0.14 to 0.83). None of these improvements exceeded the MCID for the MMSE of 1 to 3 points, however.30

 Figure 2.
Figure 2. Forest plot for the mean differences in Mini Mental State Examination scores.
APOE = apolipoprotein E; DL = DerSimonian-Laird; MCI = mild cognitive impairment; MMSE = Mini Mental State Examination.
The Clinical Dementia Rating–Sum of Boxes scale (CDR-SB) is a combined cognitive and functional scale with an MCID of 1 to 2 points (Figure 3). Overall, the CDR-SB was improved slightly with the anti-amyloid antibodies compared with placebo (MD = −0.18 points; 95% CI, −0.34 to −0.03). The only individual antibodies with a statistically significant improvement in the CDR-SB were lecanemab (MD = −0.43 points; 95% CI, −0.78 to −0.07) and donanemab (MD = −0.59 points; 95% CI, −0.86 to −0.33). Neither of these differences exceeded the MCID for the CDR-SB of 1 to 2 points, however.30
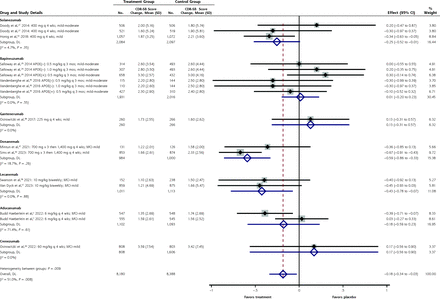
 Figure 3.
Figure 3. Forest plot for the mean differences in the Clinical Dementia Rating–Sum of Boxes scale.
APOE = apolipoprotein E; CDR-SB = Clinical Dementia Rating–Sum of Boxes scale; DL = DerSimonian-Laird; MCI = mild cognitive impairment.
A forest plot of the summary estimates of the SMDs for the 3 functional scales—the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale, the Alzheimer’s Disease Cooperative Study–Activities of Daily Living scale for patients with Mild Cognitive Impairment (ADCS-ADL-MCI) scale, and the Disability Assessment for Dementia (DAD)—is shown in Supplemental Figure 6 (forest plots for each scale separately are shown in Supplemental Figures 7-9). Overall, there was a statistically significant improvement in the combined functional scores with the anti-amyloid antibodies compared with placebo (SMD = 0.09; 95% CI, 0.05 to 0.13). Scores were also improved for aducanumab (SMD = 0.14; 95% CI, 0.06 to 0.23) and lecanemab (SMD = 0.19; 95% CI, 0.09 to 0.28) individually. None of these changes exceeded the MCID of 0.5 standardized differences, however.22,23 Forest plots of the summary estimates of the SMDs for the Dependence Scale and for the Neuropsychological Test Battery scale are shown in Supplemental Figure 10 and Supplemental Figure 11, respectively.
None of the studies reported other clinically important outcomes such as functional dependence, placement in memory care units or nursing homes, caregiver burden, or development of aggressive behaviors.
Potential HarmsOverall, there was no significant difference between treatment and control groups with regard to all-cause mortality, as shown in Supplemental Figure 12 (RR = 1.15; 95% CI, 0.85 to 1.56). One drug, bapineuzumab, was associated with a significant increase in mortality (RR = 1.76; 95% CI, 1.03 to 3.00; NNH = 102). There was no significant difference between treatment and control groups in serious adverse events, shown in Supplemental Figure 13 (RR = 1.02; 95% CI, 0.92 to 1.12).
The most frequently reported harms were ARIA-E, symptomatic ARIA-E, and ARIA-H. Those are summarized in the forest plot in Figure 4 (forest plots stratified by drug for each harm are shown in Supplemental Figures 14-16).
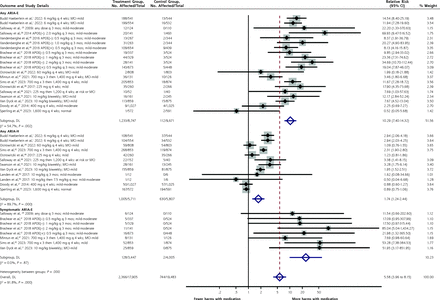
 Figure 4.
Figure 4. Forest plot for differences in any ARIA-E, any ARIA-H, and symptomatic ARIA-E.
APOE = apolipoprotein E; ARIA-E = amyloid-related imaging abnormalities of edema; ARIA-H = amyloid-related imaging abnormalities of hemorrhage; DL =DerSimonian-Laird; MCI = mild cognitive impairment. Note: Separate plots stratified by drug are given in Supplemental Figures 14-16.
Development of any ARIA-H was significantly more common overall in patients given an anti-amyloid antibody (RR = 1.74; 95% CI, 1.24 to 2.44; NNH = 13). This outcome was also significantly more likely for the 2 FDA-approved drugs, lecanemab (RR = 2.33; 95% CI, 1.44 to 3.77; NNH = 9) and aducanumab (RR = 2.94; 95% CI, 2.27 to 3.79; NNH = 8), as well as for donanemab (RR = 2.31; 95% CI, 1.90 to 2.80), than for the other antibodies.
Any ARIA-E was also significantly more common overall in treated patients (RR = 10.29; 95% CI, 7.40 to 14.3; NNH = 9). This was also true for the 2 FDA-approved drugs, aducanumab (RR = 13.1; 95% CI, 9.0 to 18.9; NNH = 3) and lecanemab (RR = 8.1; 95% CI, 4.92 to 13.3; NNH = 9), as well as for donanemab (RR = 6.5; 95% CI, 1.98 to 21.4; NNH = 7).
Finally, symptomatic ARIA-E was distinguished from any ARIA-E in some studies. Although the overall RR was significantly increased for the 3 drugs for which this outcome was reported, the absolute increase was modest (RR = 24.3; 95% CI, 9.9 to 59.9; NNH = 86). It was significantly increased for the FDA-approved drug lecanemab (RR = 52; 95% CI, 3.2 to 852; NNH = 34) and for donanemab (RR = 20.7; 95% CI, 3.1 to 138; NNH = 25), although with broad CIs.
Assessment of Heterogeneity and Publication BiasHeterogeneity across the studies was generally low, with a few exceptions. The 2 studies of aducanumab had substantial heterogeneity for CDR-SB scores (I2 = 71%) and MMSE scores (I2 = 63%), as well as for ADCS-ADL-MCI functional scale scores (I2 = 56%). Lecanemab had moderate heterogeneity with respect to the ADAS-Cog-14 score (I2 = 47%). Overall there was significant heterogeneity for the outcome of ARIA-H (I2 = 89%), with summary estimates of the relative risk for different drugs ranging from 0.82 (ponezumab) to 2.94 (aducanumab).
Funnel plots for key benefit outcomes (ADAS-Cog-11 to -14, CDR-SB, and MMSE scores) and harm outcomes (ARIA-E and ARIA-H) are shown in Supplemental Figures 17-21. These plots show no evidence of publication bias.
DISCUSSIONWe identified 19 reports of 24 studies of monoclonal antibodies targeting amyloid depositions in patients who largely had mild cognitive impairment and mild Alzheimer disease. In no case did the results of any single study, of all combined studies for an individual drug, or of all combined studies overall find a change in cognition or function that exceeded the MCID for that scale. This was also true for lecanemab and aducanumab, the only 2 FDA-approved drugs, and for donanemab, which is pending approval. For their primary outcome of the CDR-SB (MCID = 1 to 2 points),30 the studies found an improvement over placebo of only 0.43 points for lecanemab, 0.18 points for aducanumab, and 0.59 points for donanemab after 18 months of treatment.
We did find, however, that these drugs consistently cause statistically significant and potentially clinically significant increases in harms. The NNH was 13 for any ARIA-H, 9 for any ARIA-E, and 86 for symptomatic ARIA-E. The cost of these drugs is also substantial ($26,500 to $28,200 per year), and the requirement for regular magnetic resonance imaging monitoring adds considerable cost and inconvenience.
Some might argue that a longer study would find a clinically meaningful difference, but the changes we documented were so much lower than the MCIDs that this seems unlikely. For example, the improvement over placebo for the CDR-SB with lecanemab was 0.43 points after 18 months. The MCID for this scale is 1 to 2 points, so assuming a linear improvement in CDR-SB over time, it would take 3 years to get to 0.86 points and 6 years to reach 1.72 points. For aducanumab, with its improvement of only 0.18 points, it would take more than 5 years to reach even a 1-point change, again assuming linearity of effect.
It is possible that treatment earlier in the course of disease would be more beneficial. Indeed, all of the drugs approved or pending approval (lecanemab, aducanumab, and donanemab) were primarily studied in patients with mild cognitive impairment or mild Alzheimer disease, whereas most other drugs were studied in patients with mild to moderate dementia. As noted above, though, none of these drugs achieved the MCID for any benefit outcome. Also, a recent 4.5-year randomized trial of solanezumab in patients even earlier in the clinical pathway (having amyloid deposition but normal cognition) found no benefit at all.43
The FDA has previously argued that decisions about drug approvals should be based on the MCID. Lecanemab and aducanumab, however, were both approved based primarily on their effect on imaging and biomarkers, without any meaningful improvement in clinical outcomes. We feel that this is inappropriate and sets a bad precedent for the agency, not only for Alzheimer disease but also for other conditions wherein intermediate markers are easily measured but may not reliably predict clinical outcomes.
Our analysis had several limitations. All studies enrolled participants who underwent positron emission tomography scanning and/or cerebrospinal fluid analyses for amyloid, studies that are not typically done in current routine clinical practice. The included studies reported average changes on standard cognitive and functional scales, but did not report the percentage of participants achieving clinically meaningful differences in cognition or function from baseline. Such data would be more interpretable for clinicians and patients. Finally, studies had different inclusion criteria for severity of disease at baseline, which is a source of potential heterogeneity.
At best, anti-amyloid monoclonal antibodies, including those approved by the FDA, slightly slow the rate of progression of the dementia. Cognitive enhancers (donepezil, rivastigmine, galantamine, and memantine) also slow the rate of cognitive decline.52,53 Although these older drugs, as monotherapy, do not provide a benefit that exceeds the MCID, at least their safety and cost are much better than those of the newer agents. To our knowledge, no head-to-head comparisons exist, and a search of ClinicalTrials.gov performed February 2, 2023 failed to identify any planned clinical trials comparing cholinesterase inhibitors and anti-amyloid antibodies in adults with dementia.
CONCLUSIONAlzheimer disease causes tremendous suffering in those afflicted, serious burdens to their families and caregivers, and enormous costs to the health care system. Each of these groups hope for effective tools to alleviate these burdens and to extend the time of meaningful life. But our meta-analysis shows that monoclonal antibodies targeting amyloid do not provide a clinically meaningful benefit, are associated with significant harms, and come at a high cost.
FootnotesConflicts of interest: authors report none.
Read or post commentaries in response to this article.
Data sharing statement: The authors will make the spreadsheet containing the primary data, along with a data dictionary, available to other researchers who request it. Anyone using the data for further publications should involve the authors in that publication.
Received for publication May 4, 2023.Revision received September 20, 2023.Accepted for publication September 21, 2023.© 2024 Annals of Family Medicine, Inc.
Comments (0)